
How to use Agrocia
Leaflet accompanying the packaging: information for the user
Agrocia, 50 mg/mL, solution for the skin
Minoxidil
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor, pharmacist, or nurse.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If there is no improvement or the patient feels worse after 30 days, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Agrocia and what is it used for
- 2. Important information before using Agrocia
- 3. How to use Agrocia
- 4. Possible side effects
- 5. How to store Agrocia
- 6. Contents of the pack and other information
1. What is Agrocia and what is it used for
The active substance of Agrocia is minoxidil. Agrocia is used to treat male pattern baldness (androgenetic alopecia).
Male pattern baldness can be recognized by a bald spot on the top of the head (in the tonsure area).
Agrocia, 50 mg/mL, should not be used in women.
2. Important information before using Agrocia
When not to use Agrocia:
Warnings and precautions
Before starting treatment with Agrocia, the patient should discuss it with their doctor or pharmacist.
- If the patient's relatives do not have hair loss, or if the hair loss is sudden and/or partial, or if the cause of hair loss is unknown, Agrocia should not be used.
- If hair loss is associated with certain treatments (such as chemotherapy, anticancer treatment) or diseases, such as iron deficiency anemia, thyroid dysfunction, lupus (an autoimmune disease), or syphilis (a sexually transmitted infection), or severe eating disorders, or certain grooming habits, such as tight braiding or ponytails.
and (or) structure of the hair.
- If the patient is using occlusive dressings or other topical medicines on the scalp.
- If there is inflammation, irritation, or infection of the scalp, or a skin disease called psoriasis, in case of severe sunburn or skin irritation, or if the patient has a shaved scalp, Agrocia should not be used.
- Agrocia should not be used if there are any diseases or wounds on the scalp, as the medicine may be absorbed to a greater extent. This should be checked before using the medicine.
- If the patient has a history of any vascular or heart disease, as the doctor will need to monitor blood pressure and heart rate.
- If any other general or skin-related symptoms occur, treatment should be discontinued and the doctor or pharmacist consulted.
- Hands should be washed very carefully after applying the medicine to the scalp, as there is a risk of hair growth in other areas if the medicine comes into contact with them.
- If the medicine comes into contact with the eyes or mucous membranes, these areas should be rinsed with plenty of water. If necessary, an ophthalmologist should be consulted.
- Accidental ingestion of the medicine may cause severe side effects related to the cardiovascular system. Therefore, the medicine should be kept out of sight and reach of children.
- Agrocia may change the color and/or structure of the hair.
- This medicine may cause a sudden decrease in blood pressure in patients taking vasodilators (medicines used to treat cerebrovascular disorders) and medicines used to treat high blood pressure (such as guanethidine and its derivatives).
- The treated area of the scalp should not be exposed to sunlight (even on cloudy days) or lamps emitting ultraviolet radiation (UVA). It is essential to use proper protection for the treated area of the scalp.
- At the beginning of treatment with this medicine, there may be an increased loss of hair due to hair renewal. If the increased hair loss persists for more than 2 weeks, the use of Agrocia should be discontinued and the doctor consulted.
- Due to the content of ethanol (alcohol) and propylene glycol, the medicine may cause irritation and dryness of the skin.
Do not inhale the aerosol.
There have been cases of excessive hair growth on the body of infants following contact with the skin of areas where minoxidil has been applied to patients (caregivers) using topical minoxidil.
Hair growth returned to normal within a few months when the infant was no longer exposed to minoxidil.
Caution should be exercised to avoid contact between children and areas of the body where minoxidil has been applied topically.
If excessive hair growth on the body of a child is observed during the use of products containing minoxidil, a doctor should be consulted.
The use of Agrocia should be discontinued and a doctor consulted if hypotension or chest pain, rapid heart rate, fainting, or dizziness, sudden, unexplained weight gain, swelling of the hands or feet, or persistent redness or irritation of the scalp occurs.
Agrocia and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
The use of Agrocia with vasodilators (medicines used to treat cerebrovascular disorders) and medicines used to treat high blood pressure (such as guanethidine and its derivatives) may cause a sudden decrease in blood pressure.
This medicine should not be applied simultaneously with other topical medicines and hair products, such as corticosteroids, retinoids, such as tretinoin creams or ointments with occlusive effects, as they may increase the absorption of the medicine.
Betamethasone dipropionate (a medicine used to treat skin diseases) may decrease the systemic absorption of Agrocia.
When used concomitantly with cyclosporine administered systemically (a medicine that prevents organ transplant rejection), excessive hair growth may occur.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Agrocia is not recommended during pregnancy and breastfeeding.
Driving and using machines
Although there is no data to suggest that Agrocia affects the ability to drive or use machines, these activities should be avoided until it is established how the patient tolerates the medicine.
Agrocia contains ethanol and propylene glycol.
Agrocia contains 243 mg of alcohol (96% ethanol) in each mL of the solution for the skin. The medicine may cause a burning sensation on damaged skin. Due to the ethanol content, the medicine is flammable. It should not be used near an open flame, a lit cigarette, or certain devices (such as hair dryers).
Agrocia contains 520 mg of propylene glycol in each mL of the solution for the skin.
3. How to use Agrocia
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor, pharmacist, or nurse. If in doubt, the patient should consult their doctor, pharmacist, or nurse.
The recommended daily dose is 1 mL of the solution (10 sprays of the aerosol using the dosing pump) twice a day.
Agrocia stabilizes the process of male pattern baldness (androgenetic alopecia) in the tonsure area of the scalp in men. Treatment may therefore stop the progression of androgenetic alopecia. The onset and degree of response to treatment vary from patient to patient; however, individual reactions cannot be predicted due to the lack of available data.
Treatment should not be discontinued too early, as discontinuation of treatment may cause a return to the initial state of baldness before starting treatment within 3 to 4 months.
- Hands should be washed thoroughly after applying the medicine.
- Before applying the medicine, the hair and scalp should be dried thoroughly, and then the medicine should be applied, starting from the center of the treated area. The medicine should not be applied to any other part of the body, due to the risk of hair growth in those areas.
- The medicine is applied using a dosing pump equipped with a dose button and an applicator with an extended tip. Before applying the medicine, the applicator, connected to the dosing pump, should be placed in the bottle. The following instructions for attaching the applicator and placing the dosing pump in the bottle should be followed:
- Attach the applicator to the dosing pump: hold the pump firmly and press the upper part of the applicator.
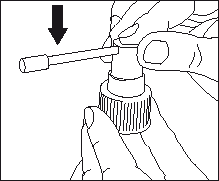
- Remove the cap from the bottle.
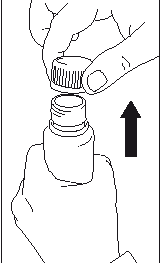
- Place the dosing pump in the bottle and screw it on tightly.
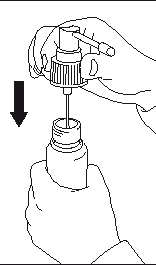
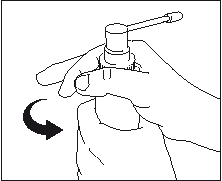
- Release the dosing pump; the dispenser is ready for use.
- Direct the applicator to the center of the bald area, press the pump once, and spread the solution over the entire area with the fingertips (with or without massaging).
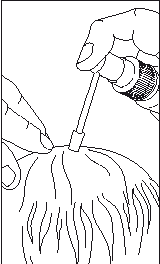
- A dose of 1 mL of minoxidil is obtained after 10 presses of the pump button.
As with other medicines, the degree of response to treatment varies from patient to patient, so it may be necessary to use the medicine for a period of 4 months to achieve noticeable hair growth. During long-term use, the effects of treatment remain unchanged over time. If no improvement in hair growth is observed after 6 months, treatment should be discontinued.
Dosing
For topical use only. DO NOT SWALLOW THE MEDICINE.
The recommended daily dose is 1 mL of the solution (10 sprays of the aerosol using the dosing pump) twice a day.
The maximum daily dose is 2 mL.
The recommended daily dose should be followed, regardless of the size of the bald area of the scalp.
If the patient feels that the effect of Agrocia is too strong or too weak, they should consult their doctor or pharmacist.
Use in children and adolescents
Agrocia should not be used in patients under 18 years of age, as its efficacy and safety in this age group have not been established.
Use in patients over 65 years of age
Agrocia should not be used in patients over 65 years of age, as its efficacy and safety in this age group have not been established.
Using more than the recommended dose of Agrocia
If a dose of Agrocia greater than the recommended dose is used, the patient should immediately consult their doctor or pharmacist.
Accidental or intentional overdose of Agrocia may cause an increase in skin-related side effects, such as itching (pruritus), dryness, irritation, and rash (acute or chronic inflammatory skin diseases).
Subjective and objective symptoms after accidental or intentional ingestion of Agrocia may include hypotension (decreased blood pressure), tachycardia (rapid heart rate), edema (swelling), and congestive heart failure (heart failure).
In case of overdose or accidental ingestion, medical help should be sought immediately, and the amount of medicine taken should be reported.
Missing a dose of Agrocia
The next dose should be applied, and then the normal treatment schedule should be resumed. A double dose should not be applied to make up for a missed dose.
Stopping treatment with Agrocia
If treatment with Agrocia is discontinued, the baldness may recur, and the patient's condition may return to what it was before treatment within 3 to 4 months.
If such symptoms occur, the patient should discuss them with their doctor.
If the patient has any further doubts about the use of this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
The patient should immediately consult their doctor if they experience any of the following symptoms, as urgent treatment may be necessary.
Swelling of the face, lips, or throat, which makes swallowing or breathing difficult. These may be symptoms of a severe allergic reaction (frequency not known, cannot be estimated from the available data).
Like all medicines, Agrocia can cause side effects, although not everybody gets them.
The most common side effects are: itching (pruritus), skin inflammation (redness), dryness, irritation, rash (inflammatory skin disease), and excessive hair growth (excessive hair growth on the skin). These effects are usually mild to moderate and reversible after discontinuation of treatment.
Very common(may affect more than 1 in 10 people):
- Headache
Common(may affect up to 1 in 10 people):
- Local skin irritation (scaling and redness)
- Contact dermatitis
- Dry skin
- Edema (swelling)
- Burning and itching of the skin
- Excessive hair growth (excessive hair growth on the skin)
- Dyspnea (breathing difficulties)
- Musculoskeletal pain
- Depression
- Acne-like rash
Uncommon(may affect up to 1 in 100 people):
- Rash (inflammatory skin disease)
- Dizziness, discomfort
- Eye irritation
- Change in hair color
- Change in hair structure
Rare(may affect up to 1 in 10,000 people):
- Changes in blood pressure and heart rate
- Transient hair loss, uneven hair growth
- Chest pain
Not known(frequency cannot be estimated from the available data)
- Allergic reactions, including angioedema (difficulty swallowing or breathing)
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Pharmacovigilance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Agrocia
The medicine should be stored out of sight and reach of children.
There are no special precautions for storage of the medicinal product.
Do not use this medicine after the expiry date stated on the bottle and carton after "EXP" and "Expiry date (EXP)". The expiry date refers to the last day of the month.
Shelf life after first opening the bottle: 30 days.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Agrocia contains
The active substance of Agrocia is minoxidil: each mL of the solution contains 50 mg of minoxidil.
One mL of the solution corresponds to 10 sprays.
The other ingredients (excipients) are: 96% ethanol, propylene glycol, and purified water.
What Agrocia looks like and contents of the pack
Agrocia is a solution for the skin. The solution is clear, colorless, or slightly yellowish, with an alcoholic odor.
Agrocia is available in an HDPE bottle with a PP cap and a PP dosing pump, in a cardboard box. The pack contains 60 mL of the solution or 120 mL of the solution (2 x 60 mL).
Marketing authorization holder
Medical Valley Invest AB
Brädgårdsvägen 28
236 32 Höllviken
Sweden
Manufacturer
Industrial Farmacéutica Cantabria S.A.
Barrio Solía, nº 30
La Concha, Villaescusa
39690 Santander, Cantabria
Spain
Date of last revision of the leaflet:08/2024
Other sources of information
Detailed information about this medicine can be found on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products http://urpl.gov.pl
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterIndustrial Farmaceutica Cantabria, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AgrociaDosage form: Solution, 20 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Aerosol, 50 mg/mlActive substance: minoxidilManufacturer: mibe GmbH Arzneimittel Sun-Farm Sp. z o.o.Prescription not requiredDosage form: Aerosol, 20 mgActive substance: minoxidilManufacturer: mibe GmbH Arzneimittel Sun-Farm Sp. z o.o.Prescription not required
Alternatives to Agrocia in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Agrocia in Ukraine
Alternative to Agrocia in Spain
Online doctors for Agrocia
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Agrocia – subject to medical assessment and local rules.







