

Androtop

Ask a doctor about a prescription for Androtop

How to use Androtop
PATIENT INFORMATION LEAFLET: USER INFORMATION
Androtop
50 mg, gel in a sachet
(Testosteronum)
You should read the contents of the leaflet before using the medicine, as it contains important information
- You should keep this leaflet, so that you can read it again if you need to.
- You should consult a doctor or pharmacist if you need advice or additional information. This medicine has been prescribed specifically for you and should not be given to others, as it may harm them, even if their symptoms are the same. If any of the side effects occur, you should inform your doctor or pharmacist. This also applies to any side effects not listed in the leaflet, see section
- 4.
Table of contents of the leaflet:
- 1. What is Androtop and what is it used for Important information before using Androtop
- 3. How to use Androtop
- 4. Possible side effects How to store Androtop Contents of the pack and other information
1. WHAT IS ANDROTOP AND WHAT IS IT USED FOR
Androtop contains testosterone, a male hormone produced physiologically in the body.
Androtop is used in adult men as testosterone replacement therapy for the treatment of
various diseases resulting from testosterone deficiency (male hypogonadism). To confirm the
disease, two independent measurements of testosterone levels in the blood and the clinical symptoms listed below are required:
- Impotence,
- Infertility,
- decreased sexual desire,
- fatigue,
- depressive moods,
- bone mass loss caused by low hormone levels,
- muscle mass loss,
- regression of male characteristics,
- inability to achieve/maintain an erection.
2. IMPORTANT INFORMATION BEFORE USING ANDROTOP
When not to use Androtop
When to exercise special caution when using Androtop
Androtop should only be used in patients with a testosterone deficiency that manifests with clear clinical symptoms (regression of secondary sex characteristics, decrease in fat-free body mass, weakness or fatigue, decreased libido, inability to achieve/maintain an erection, etc.) and has been confirmed by laboratory tests performed in the same laboratory.
Androtop is not indicated:
- for the treatment of infertility or impotence in men, due to the lack of clinical data on the use of the medicine in boys under 18 years of age, in women due to the possibility of developing male sex characteristics.
Androgens may increase the risk of prostate enlargement (benign prostatic hyperplasia) or prostate cancer. Regular prostate examinations should be performed in accordance with the doctor's recommendations before starting and during treatment.
If the patient has severe heart, liver, or kidney disease, the use of Androtop may cause serious complications, which will manifest as water retention in the body, and sometimes (congestive) heart failure.
The following blood tests should be ordered by the doctor before treatment and during treatment:
testosterone levels in the blood, complete blood count.
You should tell your doctor if you have high blood pressure or are taking medications for hypertension, as testosterone may cause an increase in blood pressure.
There have been reports of worsening breathing problems during sleep in some people treated with testosterone, especially if they have risk factors such as obesity and breathing problems.
In people with bone cancer, there may be an increase in blood or urine calcium levels. Androtop may further increase these levels. Your doctor may recommend monitoring blood calcium levels during treatment with Androtop.
In patients using testosterone replacement therapy for a long time, there may be an increase in the number of red blood cells in the blood (polycythemia). Regular blood tests are performed during treatment to check for this complication.
Androtop should be used with caution in patients with epilepsy and/or migraine, as it may exacerbate these conditions.
If very severe skin reactions occur, therapy should be re-evaluated and, if necessary, discontinued.
Certain clinical symptoms: irritability, nervousness, weight gain, frequent or prolonged erections may indicate an overdose of the medicine. These symptoms should be reported to the doctor, who will adjust the daily dose of Androtop.
Before starting treatment, the doctor should thoroughly examine the patient. Before using the medicine, it will be necessary to take blood samples on two consecutive visits to determine testosterone levels. During treatment, regular check-ups (at least once a year or twice a year in elderly patients or patients at increased risk) are performed.
Potential transfer of testosterone:
Testosterone may be transferred to another person during close and relatively prolonged skin contact with the area where the gel was applied to the patient, if the application area is not covered. This may cause the partner to experience symptoms of increased testosterone levels, such as hair growth on the face and body, and a deepened voice. This may also cause changes in the menstrual cycle in women. Wearing clothing that covers the application area or taking a shower before close contact can prevent the transfer of testosterone to another person.
The following precautions are recommended:
- washing hands with soap and water after applying the gel, covering the application area with clothing after the medicine has dried,
- taking a shower before intimate contact.
In case the gel is transferred to another person (woman or child):
- the area of skin that may have come into contact with testosterone should be washed immediately with soap and water,
- the doctor should be informed of the appearance of symptoms such as acne on the face or changes in hair growth or distribution on the body or face. To avoid putting another person at risk of accidentally receiving testosterone, a longer interval should be maintained between applying the medicine and the time of contact. During close contact, a undershirt with short sleeves should also be worn to cover the application area of Androtop or a shower should be taken or a bath before the intended contact.
A minimum interval of 6 hours is recommended between applying the gel and showering or bathing. A bath or shower can be taken from time to time after 1 to 6 hours after applying the gel, which should not significantly alter the treatment effects.
Other medicines and Androtop
You should tell your doctor about all the medicines you have taken recently, including those that are available without a prescription, especially oral anticoagulants (used to thin the blood), insulin, or corticosteroids. The use of these medicines may lead to the need to adjust the dose of Androtop.
Pregnancy and breastfeeding
Androtop is not intended for use in pregnant or breastfeeding women.
Pregnant women should avoid anycontact with the application areas of Androtop. This medicine may cause the development of unwanted male characteristics in the developing child. In case of contact with the application area of the gel, the exposed skin area should be washed with soap and water as soon as possible, as recommended above.
If the partner of a patient treated with Androtop becomes pregnant, it is necessaryto follow the recommendations for avoiding the transfer of the testosterone gel.
Driving and using machines
Androtop does not affect the ability to drive vehicles or operate machinery.
Athletes and women
Athletes and women are warned that the active substance of Androtop (testosterone) may give a positive result in doping tests.
3. HOW TO USE ANDROTOP
This medicine is intended for use only in adult men.
Androtop should always be used in accordance with the doctor's recommendations. In case of doubts, you should contact your doctor again.
The recommended dose is 5 g of gel (i.e., 50 mg of testosterone), which should be applied once a day, more or less at the same time, preferably in the morning.
The daily dose may be adjusted by the doctor individually for each patient, but should not exceed 10 g of gel per day.
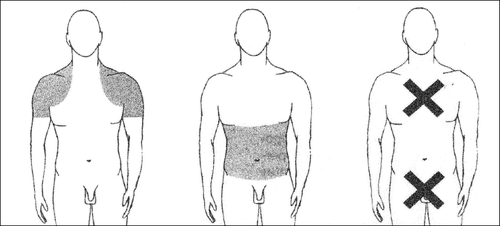
The gel should be gently spread in a thin layer on clean, dry, healthy skin of both shoulders, arms, or abdomen.
After opening the sachet, the entire contents should be removed and immediately spread in a thin layer on the skin. The gel should be allowed to dry for at least 3 to 5 minutes before dressing. After applying the gel, hands should be washed with soap and water.
Do not apply to the genital area (penis and testicles), as the high alcohol content in Androtop may cause local irritation.
Using a higher dose of Androtop than recommended
In case of using a higher dose than recommended, you should consult a doctor.
Missing a dose of Androtop
You should not take a double dose to make up for a missed dose.
Stopping treatment with Androtop
You should not stop treatment with Androtop unless the doctor recommends stopping the therapy.
In case of doubts related to the use of the medicine, you should consult a doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Androtop can cause side effects, although not everybody gets them.
Very common side effects, may occur in 10 out of 100 patients
Due to the alcohol content in Androtop, frequent application to the skin may cause skin irritation and dryness. Additionally, acne may occur.
Common side effects (between 1 and 10 out of 100 patients)
The use of Androtop may cause headaches, hair loss, pain, tenderness or swelling of the breasts, changes in the prostate, diarrhea, dizziness, increased blood pressure, mood changes, increased red blood cell count, hematocrit (percentage of red blood cells in the blood), and hemoglobin (red blood cell component that carries oxygen) levels, identified in periodic blood tests, changes in lipid levels, skin hypersensitivity reactions, skin burning, and memory disorders.
Other side effects observed during treatment with testosterone given orally or by injection:
Weight gain, changes in blood electrolyte levels, muscle pain, nervousness, depression, hostility, breathing difficulties during sleep, yellowing of the skin (jaundice), changes in liver function test results, seborrhea, changes in sexual desire, decreased sperm count, frequent or prolonged erections, urinary retention, water retention in the body, hypersensitivity reactions.
Reporting side effects
If any of the side effects occur, you should inform your doctor or pharmacist.
This also applies to any side effects not listed in the leaflet. You can also report side effects directly through the national reporting system:
Department for Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Aleje Jerozolimskie 181C
PL-02 222 Warsaw
Phone: + 48 22 49 21 301
Fax: + 48 22 49 21 309
e-mail: [email protected]
. Reporting side effects will help to obtain additional information on the safety of the medicine.
5. HOW TO STORE ANDROTOP
Storage: no special requirements.
Keep out of the reach and sight of children.
Do not use the medicine after the expiry date stated on the carton.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Androtop contains
The active substance of Androtop is testosterone. Each 5-gram sachet contains 50 mg of testosterone.
Other ingredients of the medicine are: carbomer 980, isopropyl myristate, ethanol 96% (v/v), sodium hydroxide 0.1N, purified water.
What Androtop looks like and contents of the pack
Androtop is a colorless gel packaged in 5-gram sachets.
Androtop is available in packs of 30 sachets.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Laboratoires BESINS INTERNATIONAL
3, rue du Bourg l’Abbé
75003 Paris
France
Manufacturer:
Laboratoires Besins International
or
Besins Manufacturing Belgium
13, rue Périer
Groot Bijgaardenstraat, 128
92120 Montrouge
1620 Drogenbos
France
Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
ANDROGEL:Austria, Belgium, Czech Republic, Denmark, France, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Portugal, Netherlands.
ANDROTOP GEL:Germany.
ANDROTOP:Poland, Slovenia
Date of last revision of the leaflet: 06/2015
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterBesins Manufacturing Belgium Laboratoires Besins International
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AndrotopDosage form: Gel, 25 mg/2.5 gActive substance: testosteronePrescription not requiredDosage form: Gel, 16.2 mg/gActive substance: testosteronePrescription requiredDosage form: Solution, 1000 mg/4 mlActive substance: testosteronePrescription required
Alternatives to Androtop in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Androtop in Spain
Alternative to Androtop in Ukraine
Online doctors for Androtop
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Androtop – subject to medical assessment and local rules.











