
Nebido

Ask a doctor about a prescription for Nebido

How to use Nebido
Leaflet attached to the packaging: information for the user
Nebido 1000 mg/4 ml, solution for injection
Testosterone undecanoate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so you can read it again if you need to.
- If you have any doubts, you should consult your doctor.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Nebido and what is it used for
- 2. Important information before using Nebido
- 3. How to use Nebido
- 4. Possible side effects
- 5. How to store Nebido
- 6. Contents of the pack and other information
1. What is Nebido and what is it used for
Nebido contains the active substance testosterone, a male hormone. Nebido is injected into the muscle, where it is then stored and released slowly over a certain period of time. Nebido is used in adult men as testosterone replacement therapy for various diseases that result from a lack of testosterone in the body (male hypogonadism). To diagnose the disease, two independent measurements of testosterone levels in the blood and the following clinical symptoms are required:
- impotence,
- infertility,
- decreased sex drive,
- fatigue,
- depressive moods,
- bone mass loss caused by low hormone levels.
2. Important information before using Nebido
When not to use Nebido
- -if the patient is allergic (hypersensitive) to testosterone undecanoate or any of the other ingredients of this medicine (listed in section 6);
- if the patient has androgen-dependent cancer or suspected cancer of the prostate or breast;
- if the patient has or has had a liver tumor.
Nebido is notintended for use in women.
Warnings and precautions
Before starting treatment with Nebido, you should discuss it with your doctor. You should tell your doctorif you have or have had:
- epilepsy;
- heart, kidney, or liver problems;
- migraine;
- periodic breathing pauses during sleep (apnea), as they may worsen;
- cancer, as regular blood calcium level tests may be needed;
- high blood pressure or if you are being treated for hypertension, as testosterone may increase blood pressure;
- blood clotting disorders
- coagulation disorders (such as hemophilia)
- thrombophilia (blood clotting abnormalities that increase the risk of thrombosis - the formation of blood clots in blood vessels)
- factors that increase the risk of blood clots in the vein: previous blood clots in the vein; smoking; obesity; cancer; immobilization; if someone in the patient's immediate family has had a blood clot in the leg, lung, or other organ at a young age (e.g., under 50 years); the patient's advanced age. How to recognize the occurrence of a blood clot: painful swelling of one leg or sudden change in skin color, e.g., pallor, redness, or cyanosis, sudden shortness of breath, sudden unexplained cough, which may be accompanied by coughing up blood; sudden chest pain, severe dizziness or dizziness, severe abdominal pain, sudden loss of vision. If the patient experiences any of the above symptoms, they should seek medical help immediately. If the patient has severe heart, liver, or kidney disease, using Nebido may cause serious complications, which will manifest as water retention in the body, and sometimes also (congestive) heart failure. The following blood tests should be ordered by the doctor before and during treatment: testosterone level in the blood, full blood count.
Use in patients with liver function disorders
No studies have been conducted in patients with liver function disorders. Nebido should not be prescribed if the patient has or has had a liver tumor (see section "When not to use Nebido").
Use in children and adolescents
Nebido is notintended for use in children and adolescents. There is no data on the use of the medicine in men under 18 years of age.
Use in elderly patients (over 65 years)
There is no need to change the dosage in patients over 65 years of age. (See section "Medical examination/Observation")
Muscle development and doping tests
Nebido is nota suitable means of accelerating muscle development in healthy individuals or enhancing physical performance.
Nebido may give positive results in doping tests.
Drug abuse and dependence
This medicine should always be taken according to the doctor's instructions.
Abuse of testosterone, especially when using excessive amounts of this medicine in monotherapy or in combination with other anabolic androgenic steroids, can cause serious health problems related to the heart and blood vessels (which can lead to death), mental health, and/or liver. Individuals who abuse testosterone may become dependent, which can lead to withdrawal symptoms after significant dose changes or sudden cessation of use. This medicine should not be abused, either in monotherapy or in combination with other anabolic androgenic steroids, as it is associated with serious health risks (see section 4 "Possible side effects").
Medical examination/Observation
Male hormones can accelerate the development of prostate cancer and cause prostate enlargement (benign prostatic hyperplasia). Before administering Nebido, the doctor will examine the patient to ensure that they do not have prostate cancer.
The doctor will regularly examine the prostate and breasts, especially in elderly patients, and order blood tests.
During the use of hormonal substances such as androgen compounds, cases of benign (non-cancerous) and malignant (cancerous) liver tumors have been observed.
Nebido and other medicines
You should tell your doctor or pharmacist about all medicinesyou are currently taking or have recently taken, including those that are available without a prescription. The doctor may decide to change the dosage if you are taking the following medicines:
- adrenocorticotropic hormone (ACTH) or corticosteroids (used in various diseases, such as rheumatism, arthritis, allergy, and asthma): Nebido may increase the risk of water retention in the body, especially in patients with impaired heart and liver function;
- blood-thinning tablets (oral anticoagulants, coumarin derivatives), as this may increase the risk of bleeding. The doctor will check the dosage;
- medicines used to treat diabetes. It may be necessary to adjust the dosage of the medicine that lowers blood sugar levels. Like other androgens, testosterone can enhance the effect of insulin.
You should inform your doctor if you have a blood clotting disorder, as this information is important for the doctor to decide on the administration of Nebido injection.
Nebido may also affect the results of certain laboratory tests (e.g., assessing thyroid function). You should inform your doctor or laboratory staff that you are using Nebido.
Pregnancy and breastfeeding
Nebido is not intended for use in women and should not be used in pregnant or breastfeeding women.
Effects on fertility
Systematic treatment with high doses of testosterone may reversibly inhibit or reduce sperm production (see also "Possible side effects").
Driving and using machines
Nebido does not affect the ability to drive or use machines.
Nebido contains benzyl benzoate
This medicine contains 2000 mg of benzyl benzoate in each ampoule/vial in 4 ml of solution, which corresponds to 500 mg/ml of solution.
3. How to use Nebido
The doctor will prescribe the injection of Nebido (1 ampoule/vial) very slowly into the muscle. The injection will be repeated every 10 to 14 weeks. This is enough to maintain the testosterone level at the appropriate level, without the effect of hormone accumulation in the blood.
Nebido is intended for intramuscular injection only. The person administering the injection will make sure not to administer the medicine into a blood vessel; see section "Method of administration".
Starting treatment
The doctor will assess the testosterone level in the blood before starting treatment and in its early phase.
The doctor may prescribe the next injection after 6 weeks to quickly achieve the appropriate testosterone level. This will depend on the symptoms in the patient and the testosterone levels.
Maintaining Nebido levels during treatment
The intervals between injections should be within the recommended period of 10 to 14 weeks.
The doctor will regularly check the testosterone level in the blood at the end of the interval between injections to ensure it is correct. If the level is too low, the doctor may decide to administer the medicine more frequently. If the level is too high, the doctor may decide to administer the medicine less frequently. It is essential to attend each injection appointment. Otherwise, it will not be possible to maintain the optimal testosterone level.
If you feel that the effect of Nebido is too strong or too weak, you should consult your doctor.
Using a higher dose of Nebido than recommended
Symptoms of using a higher dose of Nebido may include:
- irritability,
- nervousness,
- weight gain,
- prolonged or frequent erections.
If any of the above symptoms occur, you should consult your doctor. The doctor will decide to administer the injections less frequently or stop the treatment.
4. Possible side effects
Like all medicines, Nebido can cause side effects, although not everybody gets them.
The most common side effectsare acne and pain at the injection site.
Common side effects(may affect up to 1 in 10 people):
- increased red blood cell count;
- weight gain;
- hot flashes;
- acne;
- prostate enlargement and related problems;
- various reactions at the injection site (e.g., pain, bruising, or irritation).
Uncommon side effects(may affect up to 1 in 100 people):
- allergic reactions;
- increased appetite, changes in blood test results (e.g., increased sugar or fat levels in the blood);
- depression, emotional disorders, insomnia, anxiety, aggression, or irritability;
- headache, migraine, or tremor;
- cardiovascular disorders, high blood pressure, or dizziness;
- bronchitis, sinusitis, cough, shortness of breath, snoring, or voice problems;
- diarrhea, nausea;
- changes in liver function test results;
- hair loss or various skin reactions (e.g., itching, redness, or dryness of the skin);
- joint pain, limb pain, muscle problems (e.g., cramp, pain, or stiffness), or increased creatine phosphokinase activity in the blood;
- urinary disorders (e.g., decreased urine flow, urinary retention, need to urinate at night);
- prostate disorders (e.g., prostate dysplasia, hardening, or inflammation), changes in sex drive, testicular pain, pain, hardening, or enlargement of the breasts, or increased levels of male and female sex hormones;
- fatigue, general weakness, excessive sweating, or night sweats.
Rare side effects (may affect up to 1 in 1000 people):
- The oily solution of Nebido may enter the lungs (pulmonary oil microembolism), which can rarely cause symptoms such as: cough, shortness of breath, feeling unwell, excessive sweating, chest pain, dizziness, tingling, or fainting. These reactions may occur during or immediately after the injection and are reversible. Treatment is usually supportive, e.g., by administering additional oxygen.
After administering Nebido, anaphylactic reactions have been observed.
During the use of other testosterone-containing medicines, in addition to the above side effects, the following have also been reported: nervousness, hostility, periodic breathing pauses during sleep, various skin reactions, including dandruff and oily skin, accelerated hair growth, more frequent erections, and very rarely, yellowing of the skin and eyes (jaundice).
Using high doses of testosterone often leads to inhibition or reduction of sperm production in the body. These symptoms disappear after treatment is stopped. Testosterone replacement therapy for impaired hormonal function of the testes (hypogonadism) may rarely cause prolonged, painful erections (priapism). High doses or prolonged use of testosterone can occasionally lead to water retention in the body and edema (swelling caused by water retention).
The use of testosterone-containing products may be associated with an overall increased risk of red blood cell count, hematocrit (percentage of red blood cells in the blood), and hemoglobin concentration (a component of red blood cells that carries oxygen), observed in periodic blood tests.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Nebido
The medicine should be stored out of sight and reach of children.
There are no special precautions for storing the medicinal product.
Do not usethis medicine after the expiry date stated on the carton and label after "Expiry date". The expiry date refers to the last day of the month.
Medicines should notbe disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Nebido contains
The active substance of Nebido is testosterone undecanoate 250 mg/ml (which corresponds to 157.9 mg of testosterone).
One ampoule/vial contains 1000 mg of testosterone undecanoate (which corresponds to 631.5 mg of testosterone).
In addition, the medicine contains: benzyl benzoate and refined castor oil.
What Nebido looks like and contents of the pack
Nebido is a clear, colorless to yellowish-brown oily solution.
The pack contains:
1 ampoule made of brown glass / vial made of brown glass with 4 ml of solution for injection.
Marketing authorization holder and manufacturer
Marketing authorization holder
Grünenthal Pharmaceuticals GmbH & Co. KG
Philipp-Ott-Strasse
51373 Leverkusen
Germany
Manufacturer
Bayer AG
Müllerstrasse 178
D-13353 Berlin
Germany
or
EVER Pharma Jena GmbH
Brüsseler Strasse 18
07747 Jena
Germany
or
Grünenthal GmbH
Zieglerstrasse 6
52078 Aachen
Germany
or
Grünenthal Pharmaceuticals GmbH & Co. KG
Philipp-Ott-Straße 3
51373 Leverkusen
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
- Cyprus, Czech Republic, Greece, Denmark, Luxembourg, Malta, Poland, and Portugal: Nebido
- Austria: Nebido 1000 mg/4 ml Injektionslösung
- Belgium: Nebido 1000 mg/4 ml, oplossing voor injectie
- Bulgaria: Небидо 250 mg/ml инжекционен разтвор
- •Croatia: Nebido 1000 mg/4, ml otopina za injekciju
- Finland: Nebido 1000 mg/4 ml injektioneste, liuos
- France: Nebido 1000 mg/4 ml, solution injectable
- Germany Nebido 1000 mg Injektionslösung
- Hungary : Nebido 250 mg/ml oldatos injekció
- Iceland: Nebido 1000 mg/4 ml stungulyf, lausn
- Italy : NEBID 1000 mg/4ml soluzione iniettabile
- Lithuania : Nebido 1000 mg/4 ml injekcinis tirpalas
- Netherlands: Nebido 1000 mg/4 ml
- Norway : Nebido 1000 mg/4 ml injeksjonsvæske, oppløsning
- Romania : Nebido 1000 mg/ 4 ml soluţie injectabilă
- Slovakia: Nebido 1000 mg/4 ml injekčný roztok
- Slovenia : Nebido 1000 mg/4 ml raztopina za injiciranje
- Spain: REANDRON 1000 MG/ 4 ML SOLUCIÓN INYECTABLE
- Sweden: Nebido, 1000 mg/4 ml injektionsvätska, lösning
- United Kingdom and Ireland: Nebido 1000mg/4ml, solution for injection
Date of last revision of the leaflet:
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only:
When stored at low temperatures, the properties of this oil-based solution may change temporarily (e.g., higher viscosity, cloudiness). If the medicine is stored at low temperatures, it should be brought to room temperature or body temperature before use.
The solution for intramuscular injection should be inspected visually before use; only solutions without solid particles can be used.
The contents of the ampoule/vial should be injected intramuscularly immediately after opening.
Nebido is intended for single use only; any remaining solution should be discarded.
Method of administration:
Particular care should be taken to avoid injecting into a blood vessel.
Like all oil solutions, Nebido should be injected intramuscularly and very slowly.
Pulmonary oil microembolism can rarely lead to symptoms such as: cough, shortness of breath, feeling unwell, excessive sweating, chest pain, dizziness, tingling, or fainting. These reactions may occur during or immediately after the injection and are reversible. Treatment is usually supportive, e.g., by administering additional oxygen.
After administering Nebido, anaphylactic reactions have been observed.
Warnings:
In patients treated with testosterone, prostate and breast examinations should be performed carefully and regularly, according to the recommended procedure (rectal examination, PSA measurement). These should be performed at least once a year, and in elderly patients and those at increased risk (clinical and family factors), twice a year.
In addition to laboratory tests for testosterone levels, in patients treated with androgens in the long term, the following laboratory parameters should be monitored periodically: hemoglobin, hematocrit, liver function tests, and lipid profile.
In patients with severe heart, liver, or kidney failure or coronary artery disease, treatment with testosterone may cause serious complications, including edema, with or without congestive heart failure. In such cases, treatment should be discontinued immediately.
Instructions for preparing the OPC (One-Point-Cut) ampoule:
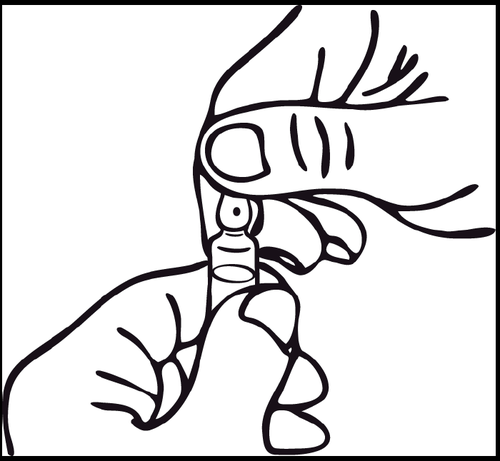
On the ampoule, below the colored mark, there is a notch that eliminates the need to score the neck. Before opening, make sure the solution from the top part of the ampoule has flowed to the bottom part. To open the ampoule, use both hands, holding the bottom part of the ampoule with one hand and breaking off the top part in the opposite direction of the colored mark.
Instructions for preparing the vial:
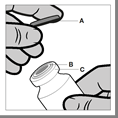
The vial is intended for single use. The contents of the vial should be injected intramuscularly immediately after drawing into a syringe. After removing the plastic cap (A), do not remove the metal ring (B) or the flanged cap (C).
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterBayer AG EVER Pharma Jena GmbH Grünenthal GmbH Grünenthal Pharmaceuticals GmbH & Co. KG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NebidoDosage form: Gel, 25 mg/2.5 gActive substance: testosteronePrescription not requiredDosage form: Gel, 50 mg/5 gActive substance: testosteronePrescription not requiredDosage form: Gel, 16.2 mg/gActive substance: testosteronePrescription required
Alternatives to Nebido in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nebido in Spain
Alternative to Nebido in Ukraine
Online doctors for Nebido
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nebido – subject to medical assessment and local rules.











