
Amidil Amihaler
Ask a doctor about a prescription for Amidil Amihaler

How to use Amidil Amihaler
Leaflet accompanying the packaging: information for the user
AMIDIL AmiHaler, 44 micrograms, powder for inhalation in hard capsules
Glycopyrronium
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is AMIDIL AmiHaler and what is it used for
- 2. Important information before using AMIDIL AmiHaler
- 3. How to use AMIDIL AmiHaler
- 4. Possible side effects
- 5. How to store AMIDIL AmiHaler
- 6. Contents of the pack and other information Instructions for using the AMIDIL AmiHaler inhaler
1. What is AMIDIL AmiHaler and what is it used for
What is AMIDIL AmiHaler
This medicine contains the active substance glycopyrronium bromide. It belongs to a group of medicines called bronchodilators.
What is AMIDIL AmiHaler used for
This medicine is used to make breathing easier for adults with breathing difficulties caused by a lung disease called chronic obstructive pulmonary disease (COPD). In COPD, there is a spasm of the airway muscles, making it difficult to breathe. This medicine works by blocking the spasm of these muscles in the lungs, making it easier for air to get in and out. Taking this medicine once a day will help alleviate the impact of COPD on daily life.
2. Important information before using AMIDIL AmiHaler
When not to use AMIDIL AmiHaler
Warnings and precautions
Before starting to use AMIDIL AmiHaler, discuss with your doctor if any of the following apply to you:
During treatment with AMIDIL AmiHalerstop using the medicine and contact your doctor immediately:
AMIDIL AmiHaler is used for the maintenance treatment of COPD. Do not use this medicine to treat sudden attacks of shortness of breath or wheezing.
Children and adolescents
Do not give this medicine to children or adolescents under 18 years of age.
AMIDIL AmiHaler and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take, including medicines similar to AMIDIL AmiHaler used to treat lung disease, such as ipratropium, oxitropium, or tiotropium (so-called anticholinergic medicines). No specific interactions have been reported when AMIDIL AmiHaler was used with other medicines used to treat COPD, such as short-acting bronchodilators (e.g., salbutamol), methylxanthines (e.g., theophylline), and/or oral and inhaled steroids (e.g., prednisolone).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. There are no data on the use of this medicine in pregnant women, and it is not known whether the active substance passes into human milk.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
AMIDIL AmiHaler contains lactose
This medicine contains lactose. If you have been told that you have an intolerance to some sugars, contact your doctor before taking this medicine.
3. How to use AMIDIL AmiHaler
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
What dose of AMIDIL AmiHaler to use
The usual dose is to inhale the contents of one capsule each day. This medicine should be used once a day, as its effect lasts for 24 hours. Do not use more than the dose prescribed by your doctor.
Elderly patients
If you are 75 years of age or older, you can use this medicine at the same dose as other adults.
When to use AMIDIL AmiHaler
Use this medicine at the same time each day to help you remember to use it. You can use this medicine at any time, before or after meals or drinks.
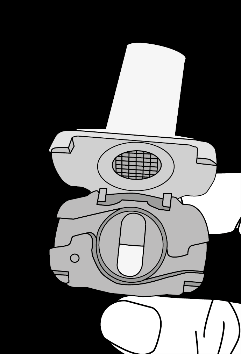
How to use AMIDIL AmiHaler
- The pack contains an inhaler and capsules (in blisters) that hold the powder for inhalation. The capsules should only be used with the inhaler provided in the pack. Store the capsules in the blisters until use.
- Do not push the capsule through the foil.
- If you start a new pack, use a new AMIDIL AmiHaler inhaler provided in the pack.
- Discard the inhaler in each pack after using all the capsules in the pack.
- Do not swallow the capsules.
- For additional information on using the inhaler, read the instructions at the end of this leaflet.
Using a higher dose of AMIDIL AmiHaler than recommended
If you have inhaled too much of this medicine or if someone else has inhaled your capsules by mistake, tell your doctor or go to the nearest hospital emergency department immediately. Show the doctor your AMIDIL AmiHaler pack. Medical attention may be necessary.
Missing a dose of AMIDIL AmiHaler
If you forget to use a dose, use it as soon as you remember. However, do not use two doses on the same day. Then, use your next dose at the usual time.
How long to use AMIDIL AmiHaler
- Use this medicine for as long as your doctor has told you.
- COPD is a long-term disease, and this medicine should be used every day, not just when you have breathing difficulties or other symptoms of COPD. If you have questions about how long to use this medicine, talk to your doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Some side effects may be serious, but they are rare(occur in less than 1 in 100 patients):
- irregular heartbeat,
- high blood sugar levels (hyperglycemia: usual symptoms include excessive thirst or hunger and frequent urination),
- rash, itching, hives, difficulty breathing or swallowing, dizziness (possible symptoms of an allergic reaction),
- swelling mainly of the tongue, lips, face, or throat (possible symptoms of angioedema). If any of these symptoms occur, tell your doctor immediately.
Some side effects may be serious, but their frequency is not known
(cannot be estimated from the available data):
- difficulty breathing with wheezing or coughing (symptoms of paradoxical bronchospasm).
Some side effects are common(occur in less than 1 in 10 patients):
- dry mouth,
- difficulty sleeping,
- runny or stuffy nose, sneezing, sore throat,
- diarrhea or abdominal pain,
- musculoskeletal pain.
Some side effects are uncommon(occur in less than 1 in 100 patients):
- difficulty urinating and pain when urinating,
- painful or frequent urination,
- palpitations,
- rash,
- numbness,
- coughing up mucus,
- tooth decay,
- feeling of pressure or pain in the cheeks or forehead,
- nosebleeds,
- arm or leg pain,
- muscle, bone, or joint pain in the chest,
- discomfort in the stomach after meals,
- throat irritation,
- feeling tired,
- weakness,
- itching,
- change in voice (hoarseness),
- nausea,
- vomiting.
In some elderly patients (over 75 years of age), headache (common) and urinary tract infection (common) may occur.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store AMIDIL AmiHaler
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the pack and blister after EXP. The expiry date refers to the last day of that month. The packaging is labeled with the expiry date and batch number (Lot/LOT). Do not store above 25°C. Store the capsules in the original blister to protect from moisture. Remove the capsules from the blister just before use. The inhaler in each pack should be discarded after using all the capsules in the pack. Do not use this medicine if you notice that the packaging is damaged or shows signs of opening. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What AMIDIL AmiHaler contains
- The active substance is glycopyrronium bromide. Each hard capsule contains 63 micrograms of glycopyrronium bromide (which corresponds to 50 micrograms of glycopyrronium). The delivered dose (the dose delivered through the inhaler mouthpiece) corresponds to 44 micrograms (µg) of glycopyrronium.
- The other ingredients are: lactose monohydrate and magnesium stearate; capsule cap: hypromellose, carrageenan (E407), indigo carmine (E132), potassium chloride, purified water; capsule body: hypromellose, carrageenan (E407), potassium chloride, purified water.
What AMIDIL AmiHaler looks like and contents of the pack
AMIDIL AmiHaler is a hard capsule with a transparent blue cap and a transparent colorless body, made of hypromellose, size 3, filled with a white or almost white powder. The pack contains a powder inhaler device for administering one dose of the medicine and perforated blisters divided into single doses. Each blister contains 10 hard capsules. The following pack sizes are available: 30x1, 60x1, or 90x1 hard capsules and one inhaler. A patient information leaflet is included in the pack. Not all pack sizes may be marketed.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A., ul. Pelplińska 19, 83-200 Starogard Gdański, tel. +48 22 364 61 01
Manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A., Oddział Medana w Sieradzu, ul. Władysława Łokietka 10, 98-200 Sieradz
Date of last revision of the leaflet
Instructions for using the AMIDIL AmiHaler inhaler
Read the Instructions for using the inhalerbefore using AMIDIL AmiHaler.
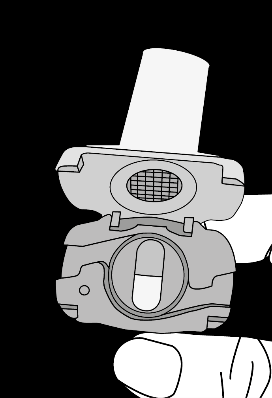
Insert the capsule Pierce the capsule by turning the base
Inhale the medicine deeply
Check if the capsule is empty
Check 2 3
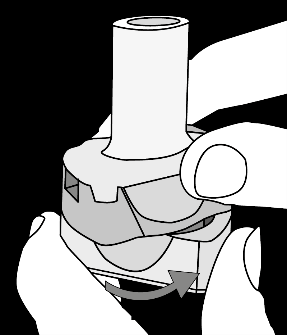
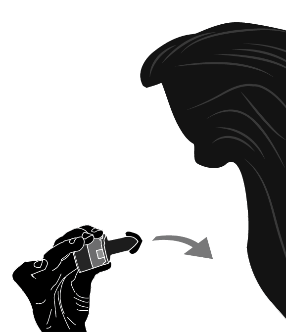
Krok 1a: Remove the cap Krok 1b: Open the inhaler Krok 1c: 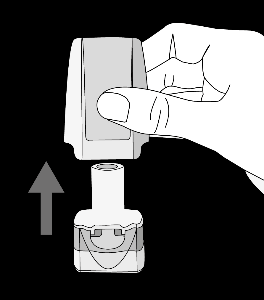 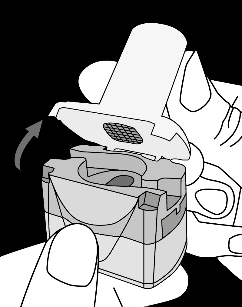 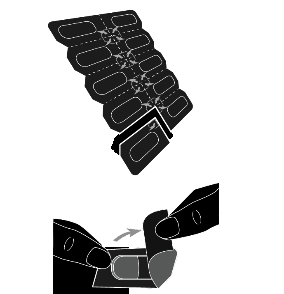 | Krok 2a: Pierce the capsule once Hold the inhaler upright. Pierce the capsule by turning the base of the inhaler 180°. You should hear the sound of the capsule being pierced. Pierce the capsule only once. 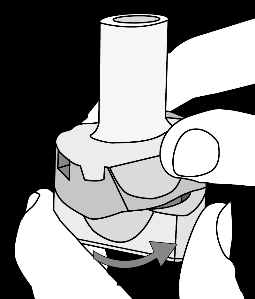 | Krok 3a: Exhale fully Before inhaling, the patient should exhale slowly and completely through the mouth outside the inhaler. Do not blow into the inhaler.   | Check if the capsule is empty Open the inhaler to check if any powder remains in the capsule. If powder remains in the capsule:
 |
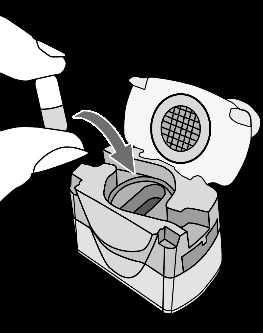
| Remove the capsule Separate one blister from the strip. Open the blister and remove the capsule. Do not push the capsule through the foil. Do not swallow the capsule. |
| ||
Krok 1e: Close the inhaler 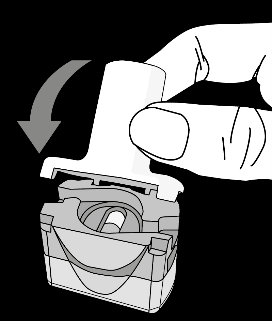 |
|
| What to do if powder remains in the capsule? This means that you did not get enough medicine. Close the inhaler and repeat steps 3a to 3c. What does coughing after inhalation mean? Coughing may occur. If the capsule is empty, you got enough medicine. What does it mean if you feel small capsule pieces on your tongue? This may happen. It is not harmful. The likelihood of the capsule breaking into small pieces increases if the capsule is pierced more than once. | Disposing of the inhaler after use Each inhaler should be discarded after using all the capsules in the pack. Ask your pharmacist how to dispose of medicines and inhalers that are no longer required. |
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A. Oddział Medana w Sieradzu
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Amidil AmihalerDosage form: Powder, 18 mcgActive substance: tiotropium bromideManufacturer: Ferrer Internacional, S.A.Prescription requiredDosage form: Aerosol, 20 mcg/dose inh.Active substance: ipratropium bromideManufacturer: Laboratorio Aldo-Union S.L. Laboratorio Echevarne, S.A.Prescription requiredDosage form: Aerosol, 20 mcg/dose inh.Active substance: ipratropium bromideManufacturer: Boehringer Ingelheim Pharma GmbH & Co. KGPrescription required
Alternatives to Amidil Amihaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Amidil Amihaler in España
Online doctors for Amidil Amihaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Amidil Amihaler – subject to medical assessment and local rules.














