
Actilise 50

Ask a doctor about a prescription for Actilise 50

How to use Actilise 50
Leaflet accompanying the packaging: information for the user
Actilyse 10, 10 mg, powder and solvent for solution for infusion
Actilyse 20, 20 mg, powder and solvent for solution for infusion
Actilyse 50, 50 mg, powder and solvent for solution for infusion
Alteplase
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Actilyse and what is it used for
- 2. Important information before using Actilyse
- 3. How to use Actilyse
- 4. Possible side effects
- 5. How to store Actilyse
- 6. Contents of the packaging and other information
1. What is Actilyse and what is it used for
The active substance of Actilyse is alteplase. It belongs to a group of medicines called thrombolytic medicines. These medicines work by dissolving blood clots that have formed in blood vessels.
Actilyse 10, 20, or 50 are used to treat a number of conditions caused by blood clots in blood vessels, including:
- heart attack caused by blood clots in the heart arteries (acute myocardial infarction)
- blood clots in the pulmonary arteries (acute massive pulmonary embolism)
- stroke caused by blood clots in the brain artery (acute ischemic stroke).
2. Important information before using Actilyse
When not to use Actilyse
- if you are allergic to the active substance alteplase or any of the other ingredients of this medicine (listed in section 6).
- if you have or have recently had a disease that increases the risk of bleeding, including:
- bleeding disorder or tendency to bleed
- severe or life-threatening bleeding in any part of the body
- intracranial bleeding or bleeding into the brain
- uncontrolled, very high blood pressure
- bacterial infections or inflammation of the heart (endocarditis), or inflammation of the membrane surrounding the heart (pericarditis)
- acute pancreatitis
- stomach or intestinal ulcers
- varices of the esophagus
- vessel disorders, such as local enlargement of an artery (aneurysm)
- certain tumors
- severe liver disease
- if you are taking "blood-thinning" medicines (oral anticoagulants), unless tests confirm that there is no clinically significant activity of such a medicine
- if you have ever undergone brain or spinal surgery
- if you have undergone major surgery in the last 3 months
- if you have recently undergone procedures involving puncture of large blood vessels
- if you have undergone external heart massage in the last 10 days
- if you have given birth in the last 10 days.
In addition, Actilyse should not be used to treat heart attack or blood clots in the pulmonary arteries:
- if you have ever had a stroke caused by bleeding into the brain (hemorrhagic stroke)
- if you have ever had a stroke of unknown cause
- if you have had a stroke caused by a blood clot in the brain artery (ischemic stroke) in the last 6 months, unless it is the stroke that this treatment is for.
In addition, Actilyse should not be used to treat stroke caused by blood clots in the brain artery (acute ischemic stroke)
- if the symptoms of ischemic stroke occurred more than 4.5 hours ago or if it is possible that the symptoms occurred more than 4.5 hours ago, because it is not known exactly when they occurred
- if the stroke causes only mild symptoms
- if there are symptoms of bleeding into the brain
- if you have had a stroke in the last 3 months
- if the symptoms are improving rapidly before Actilyse is given
- if you have had a very severe stroke
- if you have had seizures (convulsions) when the stroke occurred
- if the activated partial thromboplastin time (a blood test that checks blood clotting) is disturbed. This test may be disturbed if you have received heparin (a medicine used to "thin" the blood) in the last 48 hours
- if you have diabetes and have ever had a stroke
- if the number of platelets (thrombocytes) in the blood is very low
- if you have very high blood pressure (above 185/110), which can only be reduced by injecting medicines
- if the amount of sugar (glucose) in the blood is very low (below 50 mg/dl, which corresponds to <2.8 mmol/l)
- if the amount of sugar (glucose) in the blood is very high (above 400 mg/dl, which corresponds to > 22.2 mmol/l)
- if you are under 16 years old (patients aged ≥ 16 years see section Special warnings and precautions for use).
Special warnings and precautions for use
- if you have had an allergic reaction other than a sudden, life-threatening allergic reaction (severe hypersensitivity reaction) to the active substance alteplase or any of the other ingredients of this medicine (listed in section 6)
- if you have or have recently had any other conditions that increase the risk of bleeding, such as:
- minor injuries,
- biopsy (a procedure to take a tissue sample),
- puncture of large blood vessels,
- intramuscular injections,
- external heart massage,
- if you have ever received Actilyse,
- if you are over 65 years old,
- in elderly patients (over 80 years old), the outcome of therapy may be somewhat worse regardless of treatment, and the risk of intracranial bleeding during thrombolytic therapy may be higher compared to younger patients. In general, the benefit-to-risk ratio in elderly patients remains positive. Thrombolytic therapy in patients with acute ischemic stroke should be evaluated based on individual benefit-to-risk ratio.
- in children aged ≥ 16 years, the individual benefit-to-risk ratio should be carefully evaluated. Children aged ≥ 16 years should be treated according to adult guidelines after confirmation of arterial ischemic stroke (exclusion of stroke mimic).
Actilyse and other medicines
Tell your doctor about all medicines you are taking or have recently taken, as well as any medicines you plan to take. In particular, tell your doctor if you are currently taking or have recently taken:
- any "blood-thinning" medicines, including:
- acetylsalicylic acid,
- warfarin,
- coumarins,
- heparin,
- certain medicines used to treat high blood pressure (ACE inhibitors).
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. The doctor will use Actilyse only if the expected benefit of treatment outweighs the possible risk to the baby.
3. How to use Actilyse
Actilyse should always be prepared and administered according to the doctor's instructions. The medicine is not intended for self-administration.
Administration of Actilyse should be started as soon as possible after the onset of symptoms.
There are three indications for which this medicine can be administered:
Heart attack (myocardial infarction)
The dose used depends on body weight. The maximum dose of Actilyse is 100 mg, but it is lower for patients with a body weight below 65 kg.
The medicine can be administered in two dosing regimens:
a) 90-minute dosing regimen for patients who can start treatment within 6 hours of symptom onset
Administration consists of:
- initial injection of part of the Actilyse dose into a vein
- infusion of the remaining part of the dose over the next 90 minutes
b) 3-hour dosing regimen for patients who can start treatment between 6 and 12 hours after symptom onset
Administration consists of:
- initial injection of part of the Actilyse dose into a vein
- infusion of the remaining part of the dose over the next 3 hours
In addition to Actilyse, the doctor will administer another medicine that reduces blood clot formation. This medicine will be administered as soon as possible after the onset of chest pain.
Blood clots in the pulmonary arteries (pulmonary embolism)
The dose used depends on body weight. The maximum dose of Actilyse is 100 mg, but it is lower for patients with a body weight below 65 kg.
The medicine is usually administered as:
- initial injection of part of the dose into a vein
- infusion of the remaining part of the dose over the next 2 hours
After treatment with Actilyse, the doctor will start (or resume) treatment with heparin (a medicine used to "thin" the blood).
Stroke caused by blood clots in the brain artery (acute ischemic stroke)
Actilyse must be administered within 4.5 hours of the onset of symptoms. The sooner Actilyse is administered, the greater the benefit of treatment and the lower the risk of side effects. The maximum dose of the medicine is 90 mg, but it is lower for patients with a body weight below 100 kg.
Actilyse is administered as:
- initial injection of part of the dose into a vein
- infusion of the remaining part of the dose over the next 60 minutes
Avoid using acetylsalicylic acid in the first 24 hours after using Actilyse to treat stroke. The doctor may decide to administer heparin if necessary.
Using a higher dose of Actilyse than recommended
If the maximum recommended dose is exceeded, the risk of intracranial bleeding increases.
In cases of severe bleeding, it is recommended to transfuse fresh frozen plasma. Synthetic antifibrinolytic agents can also be administered.
If you have any further questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported in people who have used Actilyse:
Very common(occurring in more than 1 in 10 people taking the medicine)
- heart failure - treatment may need to be discontinued
- bleeding into the brain after stroke treatment - treatment may need to be discontinued
- fluid in the lungs (pulmonary edema)
- bleeding from a damaged blood vessel (such as a hematoma)
- low blood pressure (hypotension)
- chest pain (angina pectoris)
Common(occurring in less than 1 in 10 people taking the medicine)
- another heart attack
- bleeding into the brain after heart attack treatment - treatment may need to be discontinued
- cardiac arrest (cardiac arrest) - treatment may need to be discontinued
- shock (very low blood pressure) due to heart failure - treatment may need to be discontinued
- bleeding into the throat
- bleeding into the stomach or intestines, including bloody vomiting or blood in the stool, rectal bleeding
- bleeding into tissues causing purple spots (ecchymoses)
- bleeding from the urinary or reproductive organs, which may lead to blood in the urine (hematuria)
- bleeding or bruising at the injection site
Uncommon(occurring in less than 1 in 100 people taking the medicine)
- blood in the sputum (hemoptysis)
- nosebleeds
- irregular heartbeat after restoration of blood flow to the heart
- damage to the heart valves (mitral regurgitation) or the wall separating the heart chambers (ventricular septal defect) - treatment may need to be discontinued
- sudden blockage of an artery in the lungs (pulmonary embolism), brain (cerebral embolism), and in all other areas of the body (peripheral embolism)
- bleeding from the ear
- low blood pressure
Rare(occurring in less than 1 in 1,000 people taking the medicine)
- bleeding from the lungs
- bleeding into the pericardium - the membrane surrounding the heart (pericardial hematoma) - treatment may need to be discontinued
- internal bleeding into the back of the abdomen (retroperitoneal hematoma) - treatment may need to be discontinued
- formation of blood clots in blood vessels that can move to other organs (embolism). Symptoms depend on the affected organs.
- allergic reactions, such as hives and rash, difficulty breathing associated with asthma (bronchospasm), fluid under the skin and mucous membranes (angioedema), low blood pressure or shock - treatment may need to be discontinued
- bleeding into the eye
- nausea
Very rare(occurring in less than 1 in 10,000 people taking the medicine)
- severe allergic reaction (e.g., life-threatening anaphylactic reaction) - treatment may need to be discontinued
- incidents related to the nervous system, such as:
- seizures (convulsions, epileptic seizures)
- speech disorders
- confusion or delirium (severe confusion)
- anxiety accompanied by agitation
- depression
- cognitive impairment (psychosis) These disorders often occur in connection with stroke caused by blood clots or bleeding in the brain.
Frequency not known(frequency cannot be estimated from the available data)
- bleeding in internal organs, such as liver bleeding - treatment may need to be discontinued
- formation of cholesterol clots that can move to other organs (cholesterol embolism). Symptoms depend on the affected organs - treatment may need to be discontinued
- bleeding requiring blood transfusion
- vomiting
- increased body temperature (fever)
As a result of bleeding into the brain or other serious bleeding, death or permanent disability may occur.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49-21-301, fax: +48 22 49-21-309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Actilyse
Usually, there is no need for the patient to store Actilyse, as it is administered by a doctor. The medicine should be stored out of sight and reach of children.
Do not store above 25 ° C. Store in the outer packaging to protect from light.
Do not use Actilyse after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
Chemical and physical stability after preparation
Stability of the prepared solution has been demonstrated for 24 hours at a temperature of 2 ° C to 8 ° C and up to 8 hours at a temperature below 25 ° C.
Microbiological stability after preparation
From a microbiological point of view, the product should be used immediately after preparation. If the product is not used immediately, the storage time and conditions before use are the responsibility of the user, but should not exceed 24 hours in the refrigerator at a temperature of 2 ° C to 8 ° C.
6. Contents of the packaging and other information
What Actilyse contains
- The active substance of the medicine is alteplase. One vial contains 10 mg (5,800,000 IU Actilyse 10), 20 mg (11,600,000 IU Actilyse 20), or 50 mg (29,000,000 IU Actilyse 50) of alteplase.
- The other ingredients are: L-arginine, phosphoric acid (for pH adjustment), polysorbate 80.
- The solvent is water for injections.
What Actilyse looks like and contents of the pack
Actilyse is a powder and solvent for solution for infusion.
Each pack contains one vial of powder and one vial of solvent.
The vials are made of type I glass with a bromobutyl rubber stopper in a cardboard box.
Type and size of packaging:
Actilyse 10 1 vial containing 10 mg alteplase
1 vial of solvent - water for injections 10 ml
Actilyse 20
1 vial containing 20 mg alteplase
1 vial of solvent - water for injections 20 ml
Actilyse 50
1 vial containing 50 mg alteplase
1 vial of solvent - water for injections 50 ml
Not all pack sizes may be marketed.
Marketing authorization holder:
Boehringer Ingelheim International GmbH
Binger Strasse 173
D-55216 Ingelheim/Rhein
Germany
Manufacturer:
Boehringer Ingelheim Pharma GmbH & Co. KG
Birkendorfer Strasse 65
88397 Biberach/Riss
Germany
Boehringer Ingelheim France
100-104 avenue de France
75013 Paris
France
To obtain more detailed information on this medicine, please contact the local representative of the marketing authorization holder:
Poland
Boehringer Ingelheim Sp. z o.o.
Tel. + 48 22 699 0 699
Date of last revision of the leaflet: 09/2024
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Identifiability
To improve the identifiability of biological medicinal products, the name and batch number of the administered product should be clearly recorded.
Reconstitution
In order to obtain a final concentration of 1 mg alteplase per 1 ml, the entire volume of the supplied solvent should be transferred to the vial of powder. For this purpose, a transfer cannula is provided with the 20 mg and 50 mg products. For the 10 mg product, a syringe should be used.
In order to obtain a final concentration of 2 mg alteplase per 1 ml, only half of the solvent volume should be transferred (as shown in the table below). For this purpose, a syringe should be used to transfer the appropriate amount of solvent to the vial of powder.
Using aseptic technique, the powder (10 mg alteplase, 20 mg alteplase, or 50 mg alteplase) should be dissolved in water for injections according to the following scheme to obtain a concentration of 1 mg alteplase per 1 ml or 2 mg alteplase per 1 ml:
| Actilyse powder | 10 mg | 20 mg | 50 mg |
| 10 ml | 20 ml | 50 ml |
| Final concentration: | 1 mg alteplase/ml | 1 mg alteplase/ml | 1 mg alteplase/ml |
| 5 ml | 10 ml | 25 ml |
| Final concentration: | 2 mg alteplase/ml | 2 mg alteplase/ml | 2 mg alteplase/ml |
The reconstituted solution should be administered intravenously. The reconstituted solution is for immediate use.
Storage conditions - see section 5 of the leaflet.
The reconstituted solution is for single use only. Any unused solution or waste material should be disposed of in accordance with local requirements.
Instructions for reconstituting Actilyse
| 1 | Reconstitution should be performed immediately before administration.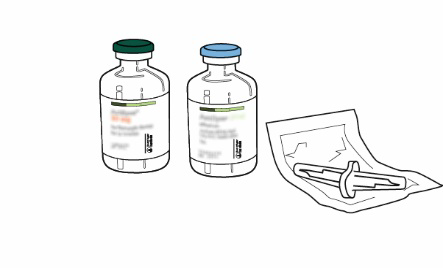 | |
| 2 | Remove the protective cap from the two vials containing water for injections and Actilyse powder by pushing it off with your thumb.  | |
| 3 | Wipe the rubber stopper of each vial with an alcohol swab.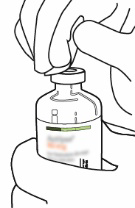 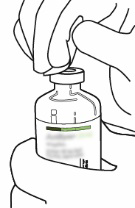 | |
| 4 | Remove the cannula* from the packaging. Do not disinfect or sterilize the cannula; it is sterile. Remove one cap.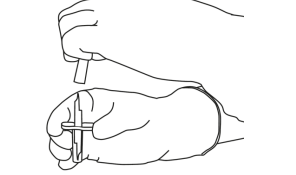 |
| 5 | Stand the vial of water for injections upright on a stable surface. Using the cannula, puncture the rubber stopper vertically from the top and center with gentle but firm pressure, without twisting.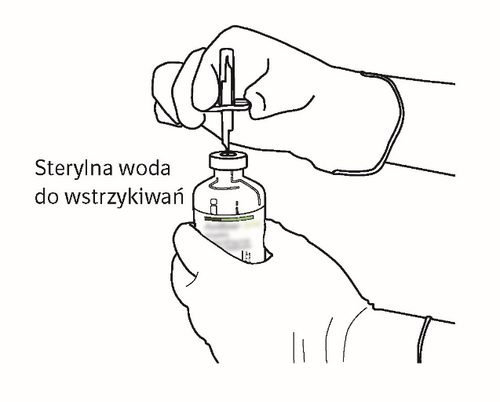 | |
| 6 | Hold the vial of water for injections and cannula using the two-winged wings. Remove the remaining cap from the top of the cannula.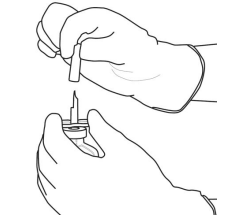 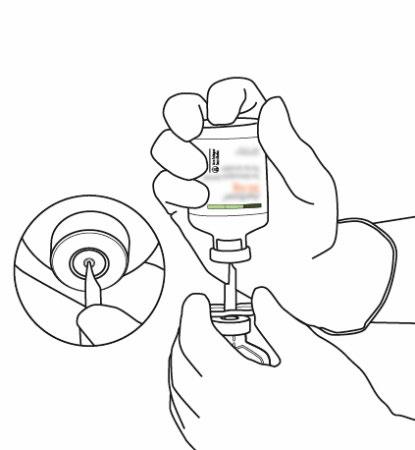 | |
| 7 | Hold the vial of water for injections and cannula using the two-winged wings. Hold the vial of powder upright over the cannula. Place the tip of the cannula on the center of the stopper. Push the vial of powder down onto the cannula, puncturing the rubber stopper vertically and gently but firmly, without twisting.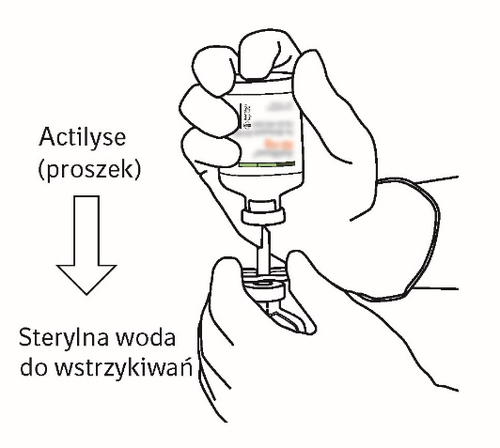 |
| 8 | Invert both vials and allow the water to flow completely into the vial of powder.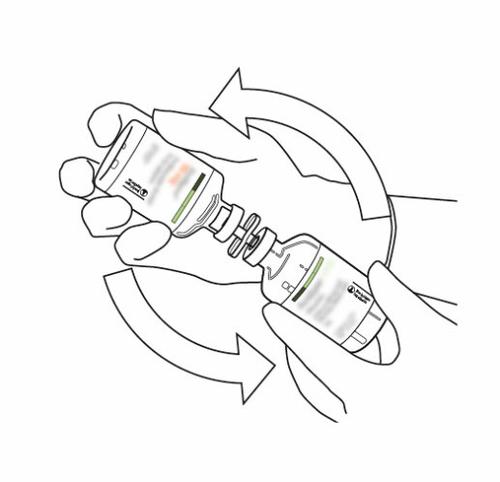 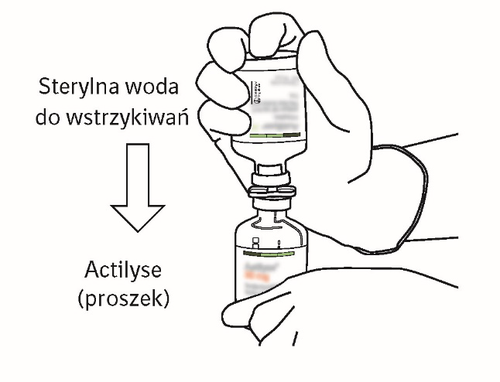 | |
| 9 | Remove the empty vial with the cannula. These items can be discarded.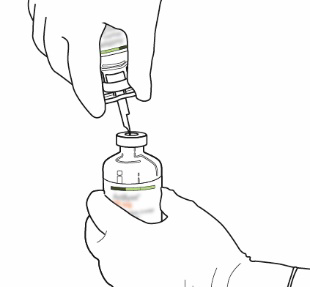 |
| 10 | Hold the vial of reconstituted Actilyse and rotate it gently in a swirling motion to dissolve any remaining powder, but do not shake, as this may cause foam to form. If bubbles appear, stand the solution and let it stand for a few minutes to allow them to disappear.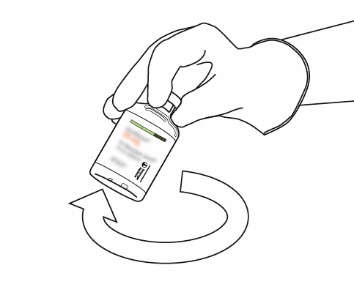 | |
| 11 | The reconstituted solution contains 1 mg/ml Actilyse. It should be clear, colorless to pale yellow, and free of particles.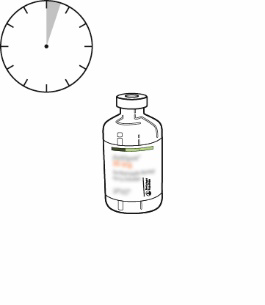 | |
| 12 | Draw up the appropriate amount using only a needle and syringe. To avoid leakage, do not use the puncture site left by the cannula. | |
| 13 | Use immediately. Discard any unused solution.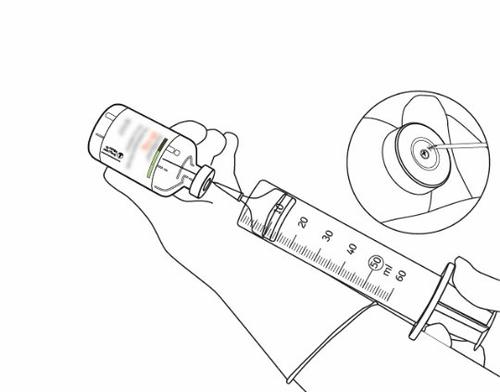 | |
(* if a cannula is provided with the kit. Reconstitution can also be performed using a needle and syringe.)
Dosage and administration
Acute myocardial infarction
Dosage
a) 90-minute (accelerated) dosing regimen for patients with acute myocardial infarction who can start treatment within 6 hours of symptom onset
For patients with a body weight ≥ 65 kg:
| Alteplase concentration | ||
| 1 mg/ml | 2 mg/ml | |
| ml | ml | |
| 15 mg in a rapid intravenous injection (bolus) with immediate administration of: | 15 | 7.5 |
| 50 mg in an intravenous infusion over the first 30 minutes, followed by immediate administration of: | 50 | 25 |
| 35 mg in a continuous intravenous infusion over 60 minutes to a maximum total dose of 100 mg | 35 | 17.5 |
For patients with a body weight <65 kg, the dose should be adjusted according to following scheme:< p>
| Alteplase concentration | ||
| 1 mg/ml | 2 mg/ml | |
| ml | ml | |
| 15 mg in a rapid intravenous injection (bolus) with immediate administration of: | 15 | 7.5 |
| ml/kg body weight | ml/kg body weight | |
| 0.75 mg/kg body weight in an intravenous infusion over the first 30 minutes (maximum 50 mg), followed by immediate administration of: | 0.75 | 0.375 |
| 0.5 mg/kg body weight in a continuous intravenous infusion over 60 minutes (to a maximum of 35 mg) | 0.5 | 0.25 |
b) 3-hour dosing regimen for patients with acute myocardial infarction who can start treatment between 6 and 12 hours after symptom onset:
For patients with a body weight ≥ 65 kg:
| Alteplase concentration | ||
| 1 mg/ml | 2 mg/ml | |
| ml | ml | |
| 10 mg in a rapid intravenous injection (bolus) with immediate administration of: | 10 | 5 |
| 50 mg in an intravenous infusion over the first hour, followed by immediate administration of: | 50 | 25 |
| ml/2 hours | ml/2 hours | |
| 40 mg in a continuous intravenous infusion over 2 hours to a maximum total dose of 100 mg | 40 | 20 |
For patients with a body weight <65 kg:< p>
| Alteplase concentration | ||
| 1 mg/ml | 2 mg/ml | |
| ml | ml | |
| 10 mg in a rapid intravenous injection (bolus) with immediate administration of: | 10 | 5 |
| continuous intravenous infusion over 3 hours to a maximum dose of 1.5 mg/kg body weight | ||
Concomitant treatment:
Concomitant anticoagulant treatment is recommended in accordance with current guidelines for patients with myocardial infarction with ST-segment elevation.
Administration:
The reconstituted solution should be administered intravenously. The reconstituted solution is for immediate use.
Acute massive pulmonary embolism
Dosage
For patients with a body weight ≥ 65 kg:
The total dose of 100 mg should be administered over 2 hours. The greatest experience has been gained with the following dosing regimen:
| Alteplase concentration | ||
| 1 mg/ml | 2 mg/ml | |
| ml | ml | |
| 10 mg in a rapid intravenous injection (bolus) over 1-2 minutes with immediate administration of: | 10 | 5 |
| 90 mg in a continuous intravenous infusion over 2 hours to a maximum total dose of 100 mg | 90 | 45 |
For patients with a body weight <65 kg:< p>
| Alteplase concentration | ||
| 1 mg/ml | 2 mg/ml | |
| ml | ml | |
| 10 mg in a rapid intravenous injection (bolus) over 1-2 minutes with immediate administration of: | 10 | 5 |
| continuous intravenous infusion over 2 hours to a maximum dose of 1.5 mg/kg body weight | ||
Concomitant treatment:
After thrombolytic treatment with Actilyse, heparin treatment should be started (or resumed) if the APTT value does not exceed twice the upper limit of normal.
Administration:
The reconstituted solution should be administered intravenously. The reconstituted solution is for immediate use.
Acute ischemic stroke
Treatment should only be carried out under the supervision of physicians experienced and trained in the field of neurology (see sections 4.3 and 4.4 of the Summary of Product Characteristics).
Treatment must be started as soon as possible within 4.5 hours of the onset of stroke (see section 4.4 of the Summary of Product Characteristics). After 4.5 hours from the onset of stroke symptoms, the benefit-to-risk ratio associated with the use of Actilyse is unfavorable, and therefore the product should not be administered (see section 5.1 of the Summary of Product Characteristics).
Dosage
The recommended total dose is 0.9 mg alteplase/kg body weight (maximum 90 mg) with an initial administration of 10% of the total dose as a rapid intravenous injection (bolus) and immediate administration of the remaining total dose as an intravenous infusion over 60 minutes.
Dosing table for the treatment of acute ischemic stroke
Body weight
Total dose
Dose in rapid injection (bolus) in mg
Dose in infusion (mg)*
40
36.0
3.6
32.4
42
37.8
3.8
34.0
44
39.6
4.0
35.6
46
41.4
4.1
37.3
48
43.2
4.3
38.9
50
45.0
4.5
40.5
52
46.8
4.7
42.1
54
48.6
4.9
43.7
56
50.4
5.0
45.4
58
52.2
5.2
47.0
60
54.0
5.4
48.6
62
55.8
5.6
50.2
64
57.6
5.8
51.8
66
59.4
5.9
53.5
68
61.2
6.1
55.1
70
63.0
6.3
56.7
72
64.8
6.5
58.3
74
66.6
6.7
59.9
76
68.4
6.8
61.6
78
70.2
7.0
63.2
80
72.0
7.2
64.8
82
73.8
7.4
66.4
84
75.6
7.6
68.0
86
77.4
7.7
69.7
88
79.2
7.9
71.3
90
81.0
8.1
72.9
92
82.8
8.3
74.5
94
84.6
8.5
76.1
96
86.4
8.6
77.8
98
88.2
8.8
79.4
100+
90.0
9.0
81.0
*administered at a concentration of 1 mg/ml over 60 minutes
Concomitant treatment
Adequate and well-controlled studies on the safety and efficacy of the above scheme in combination with concomitant administration of heparin or platelet aggregation inhibitors such as acetylsalicylic acid within the first 24 hours of stroke onset have not been conducted.
Avoid administering heparin intravenously or using platelet aggregation inhibitors such as acetylsalicylic acid within the first 24 hours after using Actilyse, due to the increased risk of bleeding.
If heparin treatment is required for other indications (e.g., prevention of deep vein thrombosis), the dose of heparin administered subcutaneously should not exceed 10,000 IU per day.
Administration
The reconstituted solution should be administered intravenously. The reconstituted solution is for immediate use.
(kg)
(mg)
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterBoehringer Ingelheim France Boehringer Ingelheim Pharma GmbH & Co. KG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Actilise 50Dosage form: Powder, 10 mgActive substance: alteplasePrescription not requiredDosage form: Powder, 20 mgActive substance: alteplasePrescription not requiredDosage form: Suppositories, 1250 IU + 15000 IUActive substance: streptokinaseManufacturer: Wytwórnia Surowic i Szczepionek Biomed Sp. z o.o.Prescription required
Alternatives to Actilise 50 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Actilise 50 in Ukraine
Alternative to Actilise 50 in Spain
Online doctors for Actilise 50
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Actilise 50 – subject to medical assessment and local rules.














