OVITRELLE 250 micrograms Injectable Solution in Pre-filled Pen

How to use OVITRELLE 250 micrograms Injectable Solution in Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ovitrelle 250micrograms solution for injection in pre-filled pen
choriogonadotropin alpha
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Ovitrelle is and what it is used for
- What you need to know before you use Ovitrelle
- How to use Ovitrelle
- Possible side effects
- Storage of Ovitrelle
- Contents of the pack and further information
1. What Ovitrelle is and what it is used for
What Ovitrelle is
Ovitrelle contains a medicine called “choriogonadotropin alpha”, which is made in a laboratory using a special DNA recombinant technique. Choriogonadotropin alpha is similar to a hormone that occurs naturally in your body, called “chorionic gonadotropin”, which is involved in reproduction and fertility.
What Ovitrelle is used for
Ovitrelle is used together with other medicines:
- To help several follicles (each containing an egg) develop and mature in women undergoing assisted reproduction techniques (a procedure that can help you become pregnant), such as “in vitro fertilization”. Other medicines will be given first to trigger the growth of several follicles.
- To help release an egg from the ovary (induction of ovulation) in women who do not produce eggs (“anovulation”) or produce very few (“oligovulation”). Other medicines will be given first to develop and mature the follicles.
2. What you need to know before you use Ovitrelle
Do not use Ovitrelle
- if you are allergic to choriogonadotropin alpha or any of the other ingredients of this medicine (listed in section 6),
- if you have a tumor in the hypothalamus or pituitary gland (both are parts of the brain),
- if you have large ovaries or large fluid-filled bags in the ovaries (ovarian cysts) of unknown origin,
- if you have vaginal bleeding of unknown cause,
- if you have ovarian, uterine, or breast cancer,
- if you have severe inflammation of the veins or blood clots in the veins (active thromboembolic disorders),
- if you have a condition that normally prevents a normal pregnancy, such as menopause or premature menopause (ovarian failure), or malformations of the sexual organs.
Do not use Ovitrelle if any of the above conditions apply to you. If you are not sure, consult your doctor before taking this medicine.
Warnings and precautions
Before starting treatment, your fertility and that of your partner must be assessed by a doctor specializing in the treatment of fertility problems.
Ovarian Hyperstimulation Syndrome (OHSS)
This medicine may increase the risk of you developing OHSS. This happens when the follicles develop too much and become large cysts.
If you notice pain in the lower abdomen, rapid weight gain, nausea, or vomiting, or difficulty breathing, do not administer the Ovitrelle injection and consult your doctor immediately (see section 4). If you develop OHSS, you may be advised not to have sex or to use a barrier contraceptive method for at least four days.
The risk of OHSS decreases if you use the usual dose of Ovitrelle and are carefully monitored throughout the treatment cycle (e.g., through blood tests to measure estradiol levels and ultrasound scans).
Multiple Pregnancy and/or Congenital Anomalies
During the use of Ovitrelle, you have a higher risk of becoming pregnant with more than one baby at the same time (“multiple pregnancy”, usually twins) than if you conceive naturally. Multiple pregnancy can lead to complications for you and your babies. During treatment with assisted reproduction techniques, the risk of multiple pregnancy is related to your age and the quality and number of eggs fertilized or embryos implanted in your body. Multiple pregnancies and certain specific characteristics of couples with fertility problems (e.g., age) may also be related to an increased risk of congenital anomalies.
The risk of multiple pregnancies decreases if you are carefully monitored throughout the treatment cycle (e.g., through blood tests to measure estradiol levels and ultrasound scans).
Ectopic Pregnancy
An ectopic pregnancy (a pregnancy outside the uterus) can occur in women with damaged fallopian tubes (the tubes that carry the egg from the ovary to the uterus). Therefore, your doctor should perform an early ultrasound scan to rule out the possibility of an ectopic pregnancy.
Abortion
During treatment with assisted reproduction techniques or stimulation of your ovaries to produce eggs, you have a higher risk of having a miscarriage than the average woman.
Blood Clotting Problems (Thromboembolic Episodes)
Consult your doctor before using Ovitrelle if you or a family member have ever had blood clots in the leg or lung, or a heart attack or stroke. You may be at a higher risk of serious blood clots or worsening of existing blood clots when receiving treatment with Ovitrelle.
Tumors of the Sexual Organs
Benign and malignant tumors of the ovaries and other sexual organs have been reported in women who have received various drug treatments for infertility.
Pregnancy Tests
If you take a pregnancy test with serum or urine after using Ovitrelle, and up to ten days later, you may get a false positive result. If you are unsure, consult your doctor.
Children and Adolescents
Ovitrelle should not be used in children and adolescents.
Other Medicines and Ovitrelle
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and Breast-feeding
Do not use Ovitrelle if you are pregnant or breast-feeding.
If you are pregnant or breast-feeding, consult your doctor before using this medicine.
Driving and Using Machines
Ovitrelle is not expected to affect your ability to drive or use machines.
Ovitrelle contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially “sodium-free”.
3. How to use Ovitrelle
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again.
How much to use
- The recommended dose of Ovitrelle is 1 pre-filled pen (250 micrograms/0.5 ml) in a single injection.
- Your doctor will explain exactly when you should administer the injection.
Using this medicine
- If you are going to administer Ovitrelle yourself, read and follow the “Instructions for use” carefully.
- Ovitrelle is administered by injection under the skin (subcutaneously).
- Each pre-filled pen is for single use only.
- Your doctor or nurse will teach you how to use the Ovitrelle pre-filled pen to inject the medicine.
- Inject Ovitrelle as your doctor or nurse has taught you.
- After the injection, dispose of the used needle safely and discard the pen.
If you use more Ovitrelle than you should
The effects of an overdose of Ovitrelle are unknown; however, there is a possibility that OHSS may occur, which is described in more detail in section 4.
If you forget to use Ovitrelle
If you forget to use Ovitrelle, contact your doctor as soon as you realize.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop using Ovitrelle and consult a doctor immediately, you may need urgent medical treatment:
- Allergic reactions such as rash, rapid or irregular heartbeat, swelling of the tongue or throat, sneezing, wheezing, or severe difficulty breathing are very rare (may affect up to 1 in 10,000 people).
- Pain in the lower abdomen, abdominal distension, or abdominal discomfort accompanied by nausea (feeling sick) or vomiting may be symptoms of OHSS. This may indicate that your ovaries have overreacted to the treatment and developed large cysts (see also section 2 under “Ovarian Hyperstimulation Syndrome”). These episodes are common (may affect up to 1 in 10 people).
- OHSS can become severe with clearly enlarged ovaries, decreased urine production, weight gain, difficulty breathing, and possible fluid accumulation in the stomach or chest. These episodes are uncommon (may affect up to 1 in 100 people).
- Severe blood clotting problems (thromboembolic episodes), sometimes independent of OHSS, are rare. These could cause chest pain, shortness of breath, stroke, or heart attack (see also section 2 under “Blood Clotting Problems”).
Other side effects
Common (may affect up to 1 in 10 people)
- Headache.
- Local reactions at the injection site, such as pain, redness, or swelling.
Uncommon (may affect up to 1 in 100 people)
- Diarrhea.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ovitrelle
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Do not use Ovitrelle if you notice visible signs of deterioration, if the liquid contains particles, or if it is not transparent.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the container and additional information
Ovitrelle Composition
- The active ingredient is chorionic gonadotropin alpha, produced by recombinant DNA technology.
- Each pre-filled pen contains 250 micrograms of chorionic gonadotropin alpha in 0.5 ml (equivalent to approximately 6,500 International Units, IU).
- The other components are mannitol, methionine, disodium hydrogen phosphate dihydrate, sodium dihydrogen phosphate monohydrate, poloxamer 188, phosphoric acid (for pH adjustment), sodium hydroxide (for pH adjustment), and water for injectable preparations.
Appearance of the product and contents of the container
- Ovitrelle is presented as a clear, colorless or slightly yellowish liquid for injection in a pre-filled pen.
- Each pen contains 0.5 ml of solution.
- It is supplied in packs of 1 pre-filled pen and 2 injection needles (one spare).
Marketing authorization holder
Merck Europe B.V., Gustav Mahlerplein 102, 1082 MA Amsterdam, Netherlands
Manufacturer responsible
Merck Serono S.p.A., Via delle Magnolie 15, 70026 Modugno (Bari), Italy.
Date of last revision of this leaflet:05/2025.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
INSTRUCTIONS FOR USE
Ovitrelle 250micrograms
Injectable solution in pre-filled pen
Chorionic gonadotropin alpha
Important information about the Ovitrelle pre-filled pen
- Read the instructions for use and the leaflet before using the Ovitrelle pre-filled pen.
- Always follow all the instructions in these instructions for use and the training provided by the healthcare professional, as they may be different from those received previously. This information will help avoid errors in treatment or needle stick infections or glass breakage injuries.
- The Ovitrelle pre-filled pen is for subcutaneous injection only.
- The Ovitrelle pre-filled pen is for single use.
- Each Ovitrelle pre-filled pen pack contains an injection needle and a spare needle.
- Only use the Ovitrelle pre-filled pen if the healthcare professional has taught you how to use it correctly.
- Store in the refrigerator.
Do notfreeze.
Do notshare the pen or needles with anyone else.
Do notuse the Ovitrelle pre-filled pen if it has fallen or if the pen is cracked or damaged, as this may cause injury.
Familiarize yourself with the Ovitrelle pre-filled pen
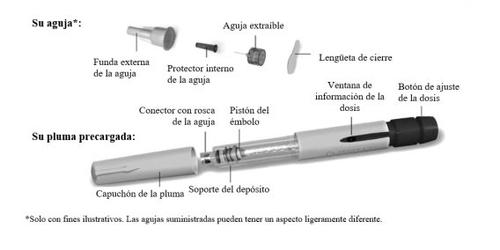
Step1 Gather materials
1.1Prepare a clean and flat surface, such as a table or countertop, in a well-lit area. |
|
1.2You will also need (not included in the package):
| |
1.3Wash your hands with water and soap and dry them well afterwards (Figure 2). | |
1.4Remove the Ovitrelle pre-filled pen from the package by hand. | |
Do notuse any utensils, as their use may damage the pen. | |
1.5Check that the pre-filled pen says Ovitrelle on it. 1.6Check the expiration date on the pen label (Figure 3). | |
Do notuse the Ovitrelle pre-filled pen if it has passed the expiration date or if the pre-filled pen does not say Ovitrelle on it. |
Step2 Prepare for injection
2.1Remove the cap from the pen (Figure 4). 2.2Check that the medicine is clear and colorless or slightly yellowish and that it does not contain particles. Do notuse the pre-filled pen if the medicine has changed color or is cloudy, as this may cause an infection. |
|
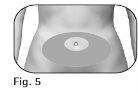
Choose an injection site: | |
2.3The healthcare professional should indicate the injection sites to use around the stomach area (Figure 5). 2.4Clean the skin at the injection site with an alcohol swab. Do nottouch or cover the skin that you have just cleaned. |
|
Step3 Attach the needle
3.1Take a new needle. Use only the single-use needles provided. 3.2Check that the outer needle shield is not damaged. 3.3Hold the outer needle shield firmly. |
|
3.4Check that the outer needle shield closure tab is not damaged or loose and that it has not passed the expiration date (Figure 6). |
|
3.5Remove the closure tab (Figure 7). |
Do notuse the needle if it is damaged or expired or if the outer needle shield or closure tab is damaged or loose. Using expired or damaged needles or outer needle shields can cause infection. Discard it in a container for sharp and pointed objects and use the other needle provided in the package.
Ask your healthcare professional if you have any questions.
3.6Screw the outer needle shield onto the threaded needle connector of the Ovitrelle pre-filled pen until you feel a slight resistance (Figure 8). Do notovertighten the needle when attaching it, as it may be difficult to remove it after injection. |
|
3.7Remove the outer needle shield by gently pulling it (Figure 9). 3.8Set it aside for later use (Figure 10). |
|
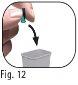
Do notdiscard the outer needle shield, as it will prevent needle stick injuries and infections when removing the needle from the pre-filled pen. 3.9Hold the Ovitrelle pre-filled pen with the needle pointing upwards (Figure 11). 3.10Carefully remove and discard the inner needle protector (Figure 12). |
|
Do notreplace the inner needle protector, as this may cause needle stick injuries and infections.
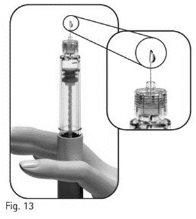
3.11Examine the needle tip carefully for one or more drops of liquid (Figure 13).
|
|
If you do not see any liquid drop on the needle tip or its surroundings when using a new pen:
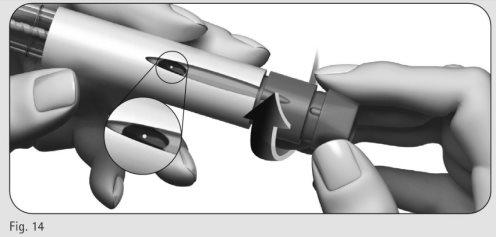
- Gently turn the dose adjustment button forward until you see a point (?) in the dose information window (Figure 14).
- You can turn the dose adjustment button backward if you have moved it beyond the point (?).

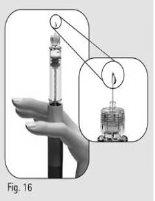

- Hold the pen with the needle pointing upwards.
- Gently tap the reservoir holder (Figure 15).
- Press the dose adjustment button completely. A drop of liquid will appear on the needle tip (Figure 16)*.
- Check that the dose information window shows “0” (Figure 17).
*Note: If you do not see any liquid, you can start again from step 1 (in this section) only once. If no drop of liquid appears the second time, contact the healthcare professional.
Step4 Select the 250 dose
4.1Gently turn the dose adjustment button forwarduntil “250”appears in the dose information window. | |
Do notpress or pull the dose adjustment button while turning it. |
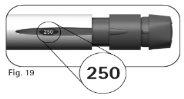
|
4.2Check that the dose information windowshows “250”(Figure 19) before proceeding to step 5 below. Contact the healthcare professional if you need help. |
|
Step5 Inject the dose
Important:inject the dose as instructed by the healthcare professional.
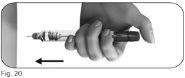
5.1Slowly insert the entire needle into the skin (Figure 20). |
|
5.2Place your thumb in the center of the dose adjustment button. Press the dose adjustment button slowly and completelyand hold it pressed to administer the injection fully (Figure 21). |
|
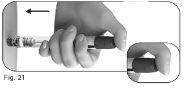
5.3Hold the dose adjustment button pressed for at least 5 seconds before removing the needle from the skin (Figure 22).
|
|
|
Do notrelease the dose adjustment button until you have removed the needle from the skin.
Step6 Remove the needle after injection
6.1Place the outer needle shield on a flat surface. |
|
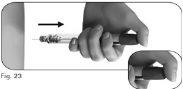
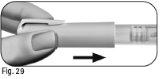
6.2Hold the Ovitrelle pre-filled pen firmly with one hand and insert the needle into the outer needle shield (Figure 24). 6.3Continue pushing the shielded needle against a firm surface until you hear a click (Figure 25). 6.4Hold the outer needle shield and unscrew the needle by turning it in the opposite direction (Figure 26). 6.5Discard the used needle safely in a container for sharp and pointed objects (Figure 27). Handle the needle with care to avoid injury. Do notreuse or share any used needle with anyone else. |
|
Step7 After injection
- Check that a complete injection has been administered:
|
|
If the dose information window shows “0”, you have completed the dose.
If the dose information window does notshow “0”, contact the healthcare professional.
Do notattempt to administer the injection a second time.
Step8 Discard the Ovitrelle pre-filled pen
Important:The Ovitrelle pre-filled pen and the provided needles are for single use.
|
Contact the healthcare professional if you have any questions.
Date of last revision of these instructions for use: 05/2025.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OVITRELLE 250 micrograms Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/0.25 ml (11 micrograms/0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for OVITRELLE 250 micrograms Injectable Solution in Pre-filled Pen
Discuss questions about OVITRELLE 250 micrograms Injectable Solution in Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions