OMNIMOXA PF 5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS

How to use OMNIMOXA PF 5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Omnimoxa PF 5 mg/ml Eye Drops Solution in Single-Dose Container
Read the entire package leaflet carefully before starting to use the medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Omnimoxa PF and what is it used for
- What you need to know before using Omnimoxa PF
- How to use Omnimoxa PF
- Possible side effects
- Storage of Omnimoxa PF
- Contents of the pack and further information
1. What is Omnimoxa PF and what is it used for
This medicine is used to treat eye infections (conjunctivitis) caused by bacteria. The active ingredient is moxifloxacin, an ophthalmic anti-infective.
Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste.
2. What you need to know before using Omnimoxa PF
Do not use Omnimoxa PF
If you are allergic (hypersensitive) to moxifloxacin, other quinolones, or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before using this medicine:
- If you experience an allergic reaction to this medicine.Allergic reactions are rare and severe reactions are very rare. If you experience any allergic reaction (hypersensitivity) or side effect, see section 4.
- If you use contact lenses –if you experience signs or symptoms of an eye infection, stop using contact lenses and wear glasses. Do not use contact lenses until the signs and symptoms of infection have resolved and you have finished using the medicine.
- Tendon inflammation and rupture have been observed in people using oral or intravenous fluoroquinolones, especially in older patients and those treated concomitantly with corticosteroids. Discontinue treatment with this medicine if you experience pain or inflammation of the tendons (tendinitis).
As with any antibiotic, long-term use of this medicine may lead to other infections.
Other medicines and Omnimoxa PF
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine, including those obtained without a prescription.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
You may experience blurred vision immediately after using this medicine. Do not drive or use machines until these effects have disappeared.
3. How to use Omnimoxa PF
Follow the administration instructions for this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults, including elderly patients and children: 1 drop in the affected eye(s), 3 times a day(morning, afternoon, and evening).
This medicine can be used in children, patients over 65 years old, and patients with kidney or liver problems. The information on the use of this medicine in newborns is very limited, and therefore, its use is not recommended in these patients.
Method of administration
This medicine should only be used as eye drops. It should only be applied to both eyes if your doctor has recommended it.
|
|
|
|
Figure 1 | Figure 2 | Figure 3 | Figure 4 |
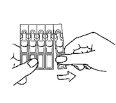
- Wash your hands.
- Take a strip of single-dose containers from the aluminum bag and separate one single-dose container. (Figure 1).
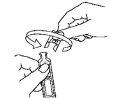
- Open the single-dose container by twisting the top end. (Figure 2).
- Tilt your head slightly backward, look up, and gently pull the lower eyelid down with your finger.
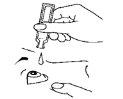
- Apply one drop into the lower conjunctival sac by gently squeezing the lower part of the container (Figure 3).
- Close your eyes slowly and press your finger against the inner corner of the eye for 2-3 minutes. This will prevent the drop from entering the tear duct and going down the throat, so most of the drop will remain in the eye (Figure 4).
- Discard the single-dose container after use.
- Put the remaining single-dose containers back in the aluminum bag and close it by folding the edge. Put the bag inside the box. If there are single-dose containers left 3 months after opening the bag, they should be discarded properly.
The contents of one single-dose container are sufficient for application in both eyes.
Do not touch the dropper tip to the eye or eyelid, surrounding areas, or other surfaces. You could infect the eye drops.
If you apply drops to both eyes, wash your hands before repeating steps 1-6 for the other eye.This will prevent the spread of infection from one eye to the other.
A new single-dose container should be used for each application.
If a drop falls outside the eye, try again.
If you have applied more moxifloxacin than you should, you can remove it by rinsing your eyes with warm water. Do not apply more drops until it is time for the next dose.
If you accidentally swallow this medicine, contact your doctor or pharmacist.
If you forget to use this medicine, continue with the next scheduled dose. Do notapply a double dose to make up for the missed dose.
If you are using other eye drops, wait at least 5 minutes between applying this medicine and the other drops.
Duration of use
The infection usually improves within 5 days. If you do not see an improvement, consult your doctor. You should continue using the drops for 2-3 days more or for the entire period indicated by your doctor.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In general, you can continue using the dropsunless the effects are severe or you experience a severe allergic reaction.
If you experience a severe allergic reaction and any of the following symptoms appear, stop using this medicine immediately and contact your doctor:swelling of the hands, feet, ankles, face, lips, mouth, or throat that may cause difficulty swallowing or breathing, rash or hives, large blisters filled with fluid, sores, and ulcers.
Common side effects(may affect up to 1 in 10 people)
- Eye effects:eye pain, eye irritation
Uncommon side effects(may affect up to 1 in 100 people)
- Eye effects:dry eye, eye itching, eye redness, inflammation or scarring of the eye surface, rupture of a blood vessel in the eye, abnormal sensation in the eye, abnormality, itching, redness, or swelling of the eyelid
- Other effects:headache, bad taste
Rare side effects(may affect up to 1 in 1,000 people)
- Eye effects:corneal disorder, blurred or reduced vision, conjunctival inflammation or infection, eye fatigue, eye swelling
- Other effects:vomiting, nose discomfort, sensation of a lump in the throat, decreased iron in the blood, abnormal liver function test values, abnormal sensation on the skin, pain, throat irritation
Frequency not known(cannot be estimated from the available data)
- Eye effects:eye infection, clouding of the eye surface, corneal swelling, deposits on the eye surface, increased pressure in the eye, scratch on the eye surface, eye allergy, eye discharge, increased tear production, sensitivity to light
- Other effects:shortness of breath, irregular heartbeat, dizziness, increased allergic symptoms, itching, rash, redness of the skin, nausea, and hives
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Omnimoxa PF
Keep out of sight and reach of children.
Store the single-dose containers in the aluminum bag and in the box to protect them from light.
Do not use the eye drops after the expiration date stated on the bottle and carton after "EXP". The expiration date refers to the last day of the month indicated.
Do not use for more than 3 months after opening the aluminum bag.
Once opened, the contents of the single-dose container should be used immediately and cannot be stored. The remaining solution in the single-dose container should be discarded after application.
Medicines should not be disposed of via wastewater or household waste. Dispose of the containers and medicines you no longer need at the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Omnimoxa PF
- The active ingredient is moxifloxacin. One milliliter of eye drops contains 5 mg of moxifloxacin (as moxifloxacin hydrochloride, 5.45 mg). One drop of eye drops contains 160 micrograms of moxifloxacin.
- The other ingredients are: sodium chloride, boric acid, hydrochloric acid, and/or sodium hydroxide (to adjust the pH) and water for injections.
Appearance and packaging of the product
This medicine is a liquid (clear, pale yellow solution).
One single-dose container contains 0.4 ml.
Omnimoxa PF is available in packs of 10 or 30 single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
OmniVision GmbH
Lindberghstraße 9
82178 Puchheim
Germany
Manufacturer
Pharma Stulln GmbH
Werksstrasse 3
92551 Stulln
Germany
You can obtain further information about this medicine by contacting the local representative of the marketing authorization holder:
OmniVision Farma España S.L.
C/ Josep Irla i Bosch, 1-3
Pl: 6 Pt: 2
08034 Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
AustriaMoxifloxacin Stulln sine 5 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis
GermanyMoxifloxacin Stulln sine 5 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis
GreeceMOFLOTREX UD 5 mg/ml Οφθαλμικ?ς σταγ?νες, δι?λυμα σε περι?κτη μ?ας δ?σης
ItalyMoxidrop
NetherlandsMoxifloxacine Stulln zonder conserveermiddel 5 mg/ml oogdruppels, oplossing in verpakking voor éénmalig gebruik
PolandMoxifloxacinum Stulln
SpainOmnimoxa PF 5 mg/ml colirio en solución en envase unidosis
Date of the last revision of this package leaflet:09/2024.
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OMNIMOXA PF 5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Omnivision GmbhPrescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Novartis Farmaceutica S.A.Prescription required
Online doctors for OMNIMOXA PF 5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about OMNIMOXA PF 5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions