NATPAR 25 micrograms/dose powder and solvent for injectable solution


How to use NATPAR 25 micrograms/dose powder and solvent for injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Natpar 25micrograms/dose powder and solvent for solution for injection
Natpar 50micrograms/dose powder and solvent for solution for injection
Natpar 75micrograms/dose powder and solvent for solution for injection
Natpar 100micrograms/dose powder and solvent for solution for injection
Parathyroid hormone
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only, do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Natpar and what is it used for
- What you need to know before you use Natpar
- How to use Natpar
- Possible side effects
- Storage of Natpar
- Contents of the pack and other information
- Instructions for use
1. What is Natpar and what is it used for
What is Natpar?
Natpar is a hormonal supplement for adults with underactive parathyroid glands, a condition called "hypoparathyroidism".
Hypoparathyroidism is a disease caused by low levels of parathyroid hormone, which is produced by the parathyroid glands in the neck. This hormone controls the amount of calcium and phosphate in the blood and urine.
If your parathyroid hormone levels are too low, you may have low blood calcium levels. Low calcium levels can cause symptoms in various parts of the body, such as bones, heart, skin, muscles, kidneys, brain, and nerves. To see a list of symptoms of low calcium levels, see section 4.
Natpar is a synthetic form of parathyroid hormone that helps maintain calcium and phosphate levels in your blood and urine at a normal level.
2. What you need to know before you use Natpar
Do not use Natpar
- if you are allergic to parathyroid hormone or any of the other ingredients of this medicine (listed in section 6).
- if you are receiving or have received radiation therapy to the bones
- if you have bone cancer or other cancer that has spread to the bones
- if you have a higher risk of developing a type of bone cancer called osteosarcoma (for example, if you have Paget's disease or other bone diseases)
- if a blood test shows that you have unexplained increases in bone alkaline phosphatase
- if you have pseudohypoparathyroidism, a rare condition in which the body does not respond properly to parathyroid hormone produced by the body
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Natpar.
If you are being treated with Natpar, you may experience side effects related to low or high calcium levels in the blood (see these side effects in section 4).
These effects are more likely to occur:
- when starting treatment with Natpar;
- if you change the dose of Natpar;
- if you miss one of the daily injections;
- or if you stop using Natpar for a short or long period.
You may be given medicines to treat these side effects or to help prevent them, or you may be asked to stop taking some of the medicines you are taking. These medicines include calcium or vitamin D.
If your symptoms are severe, your doctor may give you additional medical treatment.
Your doctor will check your calcium levels. You may need to change the dose of Natpar or stop the injections for a short period.
Tests and procedures
Your doctor will monitor how you respond to treatment:
- during the first 7 days after starting treatment and
- if the dose is changed.
This will be done through tests that measure calcium levels in the blood or urine. Your doctor may ask you to change the amount of calcium or vitamin D you take (in any of its forms, including calcium-rich foods).
Talk to your doctor or pharmacist before using Natpar if you have kidney stones.
Children and adolescents
Natpar must not be used in children and adolescents under 18 years.
Other medicines and Natpar
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including:
- digoxin, also known as digitalis, a heart medicine
- medicines for the treatment of osteoporosis, called bisphosphonates, such as alendronic acid
- medicines that may affect calcium levels in the blood, such as lithium or certain medicines used to increase the amount of urine (diuretics).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. There is limited information on the safety of Natpar in pregnant women. It has been shown that Natpar passes into breast milk in rats, but it is not known if it passes into breast milk in humans.
Your doctor will decide whether it is necessary to start treatment with Natpar. Your doctor will also decide whether you should continue using this medicine if you become pregnant or start breastfeeding during treatment.
The effect of Natpar on fertility is not known.
Driving and using machines
Natpar has no influence on the ability to drive and use machines. However, hypoparathyroidism itself may affect your ability to concentrate. If your ability to concentrate is affected, do not drive or use machines until it has improved.
Natpar contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose. This means that it is essentially "sodium-free".
3. How to use Natpar
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again. Your doctor or nurse will teach you how to use the Natpar pen.
Natpar is given as a subcutaneous injection (under the skin) every day, using a pen to help inject the medicine.
In this leaflet, the "reusable Natpar pen" is referred to as the "Natpar pen" or "pen".
Dose
The recommended starting dose of Natpar is 50 micrograms per day.
- However, your doctor may tell you to start with 25 micrograms per day based on the results of a blood test.
- After 2 to 4 weeks, your doctor may adjust the dose.
The dose of Natpar varies from person to person. You may need between 25 and 100 micrograms of Natpar per day.
Your doctor may tell you to take other medicines, such as calcium or vitamin D supplements, while you are using Natpar. Your doctor will tell you how much to take per day.
How to use the pen
Read the "Section 7. Instructions for use"of this leaflet before using the pen.
Do not use the pen if the solution is cloudy or colored, or if it contains visible particles.
Before using the pen for the first time, you need to mix the medicine.
Once you have mixed the medicine, the Natpar pen is ready to use and the medicine can be injected under the skin of the thigh. The next day, inject into the other thigh and continue alternating between the two.
Each time you receive a dose of Natpar, it is strongly recommended that you write down the name and batch number of the medicine, in order to keep a record of the batches used.
Duration of treatment
Continue using Natpar for as long as your doctor tells you to.
If you use more Natpar than you should
If you accidentally inject more than one dose of Natpar in a single day, contact your doctor or pharmacist immediately.
If you forget to use Natpar
If you forget to use Natpar (or cannot inject it at the usual time), inject it as soon as you can but do not inject more than one dose on the same day.
Give the next dose of Natpar at the usual time the next day. You may need to take more calcium supplements if you have signs of low calcium levels in the blood; see symptoms in section 4.
Do not inject a double dose to make up for forgotten doses.
If you stop using Natpar
Talk to your doctor if you want to stop using Natpar.
If you have any other questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
The following serious side effects may occur with the use of Natpar:
- Very common: high calcium levels in the blood, which may occur more often when starting treatment with Natpar.
- Very common: low calcium levels in the blood; this may occur more often if you stop using Natpar suddenly.
Symptoms related to high or low calcium levels are listed below. If you experience any of these side effects, talk to your doctor immediately.
Other side effects:
Very common(may affect more than 1 in 10 people):
- headache*,†
- tingling and numbness in the skin†
- diarrhea*,†
- nausea and vomiting*
- joint pain*
- muscle spasms†
Common(may affect up to 1 in 10 people):
- nervousness or anxiety†
- sleep problems (daytime sleepiness or difficulty sleeping at night)*
- rapid or irregular heartbeat*,†
- high blood pressure*
- cough†
- stomach pain*
- muscle twitching or cramps†
- muscle pain†
- neck pain†
- arm and leg pain
- increased calcium levels in the urine*
- need to urinate frequently†
- fatigue and lack of energy*
- chest pain
- redness and pain at the injection site
- thirst*
- antibodies (produced by your immune system) against Natpar
- in blood tests, your doctor may see decreased levels of vitamin D and magnesium†
Frequency not known(cannot be estimated from the available data):
- allergic reactions (hypersensitivity), such as: swelling of the face, lips, mouth, or tongue; difficulty breathing; itching; rash; hives
- Seizures (epileptic fits) due to low calcium levels in the blood†
*These side effects may be related to high calcium levels in the blood.
†These side effects may be related to low calcium levels in the blood.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Natpar
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the cartridge and on the carton after EXP. The expiry date is the last day of the month shown.
Before mixing
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Keep the cartridge in its pen and in the outer packaging to protect it from light.
After mixing
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Keep the pen with a mixed cartridge perfectly closed to protect the cartridge from light.
- Do not use this medicine for more than 14 days after it has been mixed.
- Do not use this medicine if it has not been stored correctly.
- Before putting a new needle on the Natpar pen, check that the solution is clear and colorless. It is normal to see small bubbles. Do not use this medicine if it has become cloudy, has changed color, or contains visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Natpar Composition
The active ingredient is parathyroid hormone (rDNA).
It comes in cartridges with 4 different concentrations (each cartridge contains 14 doses):
Natpar 25 micrograms
Each dose contains 25 micrograms of parathyroid hormone in 71.4 microliters of solution after reconstitution.
Natpar 50 micrograms
Each dose contains 50 micrograms of parathyroid hormone in 71.4 microliters of solution after reconstitution.
Natpar 75 micrograms
Each dose contains 75 micrograms of parathyroid hormone in 71.4 microliters of solution after reconstitution.
Natpar 100 micrograms
Each dose contains 100 micrograms of parathyroid hormone in 71.4 microliters of solution after reconstitution.
The other components in the cartridge (for all concentrations) are:
In the powder:
- sodium chloride
- mannitol
- citric acid monohydrate
- sodium hydroxide (for pH adjustment)
In the solvent:
- metacresol
- water for injectable preparations
Appearance of the Product and Container Contents
Each Natpar cartridge contains the medicine in powder form along with a solvent to prepare an injectable solution. The cartridge is made of glass, with a rubber stopper at the top. The cartridge is inside a plastic cartridge holder.
Natpar is available in a package with 2 cartridges in their holders.
The color of the box and the cartridge indicates the concentration of Natpar:
Natpar 25 micrograms/dose
Purple cartridge.
Natpar 50 micrograms/dose
Red cartridge.
Natpar 75 micrograms/dose
Gray cartridge.
Natpar 100 micrograms/dose
Blue cartridge.
Marketing Authorization Holder and Manufacturer
Shire Pharmaceuticals Ireland Limited
Block 2 & 3 Miesian Plaza
50 – 58 Baggot Street Lower
Dublin 2
Ireland
Tel: +44(0)1256 894 959
E-mail: [email protected]
Date of Last Revision of this Leaflet: .
This medicine has been authorized with a «conditional approval». This type of approval means that more information on this medicine is expected.
The European Medicines Agency will review the new information on this medicine at least once a year and this leaflet will be updated as necessary.
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Instructions for Use
This guide has been designed to help you prepare, inject, and store your Natpar pen.
These instructions are divided into 5 stages
Get to Know the Parts of Your Natpar Pen and the Natpar Medicine |
Preparation and Mixing of Natpar |
Preparation of the Natpar Pen |
Administration of the Daily Dose |
Storage of the Medicine |
If you need help at any time, contact your doctor, pharmacist, or nurse.
You can also contact Shire at phone +44(0) 1256 894959 or by email at [email protected].
What You Need to Know Before You Start
- DO NOT use your Natpar pen until your doctor or nurse has shown you how to do it.
- Use these instructions for use every time you mix the medicine, prepare the pen, or administer an injection, so you do not forget to carry out any of the steps.
- A new needle must be placed on the pen every day.
- A new cartridge must be prepared once every 14 days.
- DO NOT use this medicine if you notice that it has become cloudy, has changed color, or contains visible particles.
- Always keep the cartridge in the refrigerator (between 2°C and 8°C).
- DO NOT freeze the cartridge.
- DO NOT use a cartridge that has been frozen.
- Discard all mixed cartridges that are more than 14 days old.
- Administer your dose only once a day.
- To clean the Natpar pen, wipe the outside of the pen with a damp cloth. DO NOT put the pen in water, nor wash it or clean it with any liquid.
- Discard the used Natpar cartridge and the used needles according to the instructions of your doctor, pharmacist, or nurse.
- Your Natpar pen can be reused for a maximum of 2 years.
Get to Know the Parts of Your Natpar Pen and the Natpar Medicine |
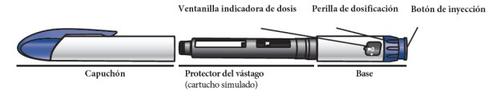
Get to Know the Parts of the Natpar Pen
Parts of the Natpar pen
Note:the needle guard (simulated cartridge) protects the needle during shipping from the factory. Discard the needle guard when you are ready to use the pen.
|
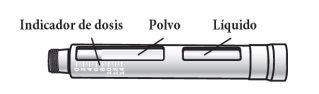
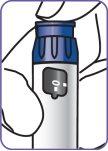
The Natpar cartridge The Natpar cartridge contains the medicine in powder form and the solvent to mix it. You must mix the powder and the solvent in the cartridge before using the Natpar pen.
|
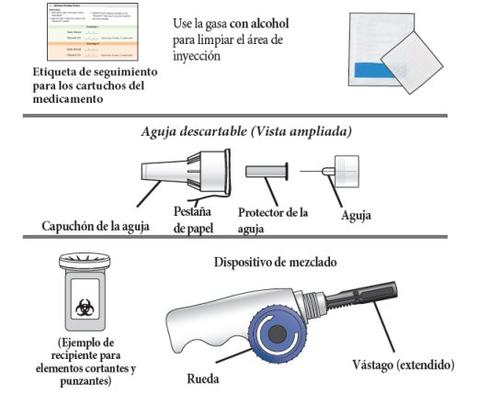
Other necessary items: Note:alcohol swabs, injection needles, and a sharps container are not included in the package. The medication tracking label is inside these instructions for use.
|
Preparation and Mixing of Natpar | ||
You must mix Natpar before you can use it. Once the medicine is mixed, it can be used for a maximum of 14injections (14doses). If this is the first time you are using Natpar yourself, your doctor or nurse will guide you through the process of mixing the Natpar cartridge. | ||
1. | When preparing to administer a dose, make sure to remove your Natpar cartridge from the refrigerator. Note:you must keep the cartridge in the refrigerator at all times, except when preparing and injecting the medicine. |
|
|
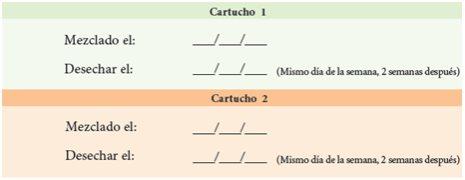
2. | Complete the dates on the medication tracking label. |
Medication Tracking Label Instructions:
|
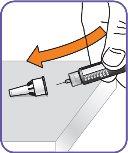
3. | Remove the paper tab from the needle cap. |
|
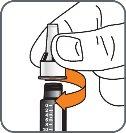
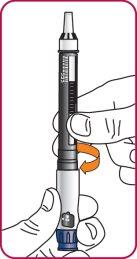
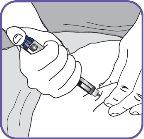
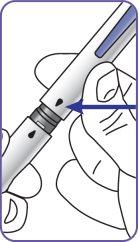
4. | Screw the pen needle onto the cartridge in a clockwise direction.
|
|
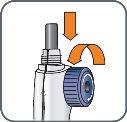
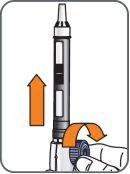
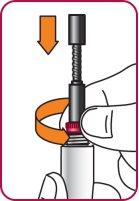
5. | Turn the mixing device wheel counterclockwise to lower the plunger, if it is not already down. |
|
|
| |
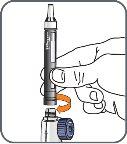
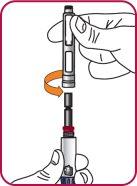
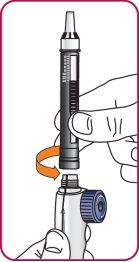
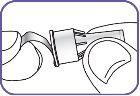
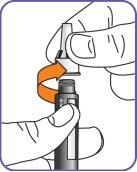
6. | Screw the Natpar cartridge onto the mixing device in a clockwise direction.
|
|
7. | With the needle cap facing up,slowly turn the wheel clockwise until the stoppers inside the cartridge no longer move and the wheel turns freely.
|
|
8. | Make sure the stoppers appear as in the image and remain together. |
|
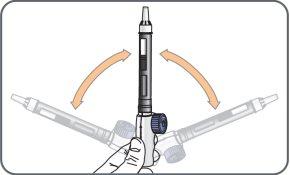
9. | Hold the mixing device with the cartridge connected, with the needle facing up, and gently move the cartridge from side to side (from the 9 to 3 o'clock position) about 10 times, to dissolve the powdercontained in the cartridge.
Check the solution before administering each daily dose. If after 5 minutes the solution is cloudy, contains visible particles, or is not colorless, do not use the medicine. Contact your doctor, pharmacist, or nurse.It is normal to see small bubbles. |
|
Preparation of the Natpar Pen | ||
You will prepare the Natpar pen onceevery 14days | ||
1. | Take the pen and remove the cap. Save the cap for later use. |
|
2. | Unscrew the needle guard (simulated cartridge) or the empty medicine cartridge counterclockwise and discard it in a sharps container. |
|
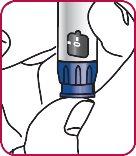
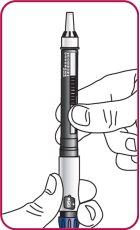
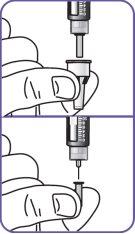
3. | Press the injection button. A "0" should be visible, aligned with the notch in the dose indicator window. If the "0" is not visible in the indicated position, press the injection button until it is aligned. |
|
4. | Lower the plunger. If the plunger is extended, turn the dark red ring counterclockwise to lower it. Do not overtighten the ring. |
|
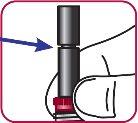
5. | Check the plunger. It should have a small groove if the procedure was done correctly. |
|
6. | Unscrew the cartridge from the mixing device counterclockwise and support the mixing device. |
|
7. | Connect the cartridge to the pen. Lift the pen base and hold it with the plunger facing up. |
|
8. | With the needle cap facing up, screw the cartridge onto the pen in a clockwise direction until there is no space between the cartridge and the pen. |
|
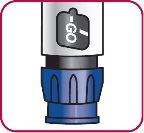
9. | Primining the Natpar Pen Turn the dose dial clockwise until the word "GO" is aligned with the notch in the dose indicator window. |
|
10. | Hold the pen with the needle cap facing up. |
|
11. | Press the injection button on a flat surface, such as a table, until the "0" is aligned with the notch in the dose indicator window.
|
|
Administration of the Daily Dose | ||
NOTE: if you have just finished mixing the medicine and preparing the pen and the pen needle is in place, go directly to “Before injecting the daily dose” (step 6 of this section) to see the instructions on how to inject yourself with the Natpar pen. If you need help at any time, ask your doctor or nurse. | ||
1. | Wash and dry your hands. | |
2. | Gather the following items:
Note:you must keep the mixed cartridge, inside the pen, in the refrigerator at all times, except when preparing and injecting the medicine. | |
3. | Check the cartridge. Remove the cap from the Natpar pen. The mixed cartridge should be inside. |
|
4. | Before placing a new needle on your pen, check the following:
If the liquid is not clear, colorless, or has visible particles, do not use this medicine. Contact your doctor, pharmacist, or nurse. You will need to prepare a new Natpar cartridge if:
or
| |
5. | Placing a new needle.
|
|
6. | Before injecting the daily dose.
| |
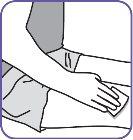
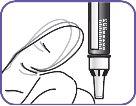
7. | Clean the injection area on the thigh with an alcohol swab. Inject into alternate thighs each day. |
|
Make sure the needle cap is pointing downwards at all times during steps 8 to 17. | ||
8. | Hold the Natpar pen with the needle tip pointing downwards.
|
|
9. | Hold the pen so that you can see the dose indicator window. |
|
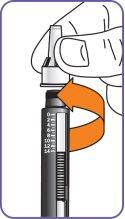
10. | Turn the dose dial until the word "GO" is aligned with the notch in the dose indicator window. DO NOTturn the dose dial beyond "GO".
Check the dose indicatoron the cartridge to see if there is any dose left, or check the date on "Discard on" on the medication tracking labelto see if more than 14days have passed. |
|
11. | Gently tap the cartridge 3to 5times. This moves any air bubbles away from the needle. |
|
12. | Prepare the pen needle for injection. Without unscrewing,
|
|
13. | Hold the pen so that you can see the word "GO" in the dose indicator window, with the needle pointing downwards. |
|
14. | Read steps 15, 16, and 17carefully before injecting the medication. | |
15. | Insert the needle fully into the thigh (you can pinch a skin fold if your doctor or nurse tells you to). Make sure you can see the word "GO" in the window. |
|
16. | Press the injection button until the "0" is aligned with the notch in the dose indicator window. You should see and feel the dose dial return to "0". Count slowly to 10. |
|
Important note about injection: To avoid administering an insufficient dose, you must keep the needle in the skin for 10 seconds AFTERpressing the injection button. |
| |
17. | Remove the needle from the thigh.
| |
18. | Carefully replace the exposed needle with the large needle cap, lifting it with a lever motion.
|
|
19. | Unscrew the needle cap (with the pen needle inside) counterclockwise while holding the cartridge.
|
|
20. | Discard the used needle in a sharps container. Ask your doctor, pharmacist, or nurse how to properly dispose of a filled sharps container. |
|
21. | Replace the cap on the pen.
|
|
22. | Store the Natpar pen in the refrigerator. |
Medication storage |
Natpar cartridges and any pen with a mixed cartridge must always be stored in the refrigerator (between 2°Cand 8°C). | ||
|
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NATPAR 25 micrograms/dose powder and solvent for injectable solutionDosage form: INJECTABLE, 100 µgActive substance: parathyroid hormoneManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription requiredDosage form: INJECTABLE, 50 µgActive substance: parathyroid hormoneManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription requiredDosage form: INJECTABLE, 75 µgActive substance: parathyroid hormoneManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription required
Online doctors for NATPAR 25 micrograms/dose powder and solvent for injectable solution
Discuss questions about NATPAR 25 micrograms/dose powder and solvent for injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions