
MIRTAZAPINE TEVA-RATIOPHARM 30 mg ORALLY DISINTEGRATING TABLETS

How to use MIRTAZAPINE TEVA-RATIOPHARM 30 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Mirtazapine Flas Teva-ratiopharm and what is it used for
- What you need to know before taking Mirtazapine Flas Teva-ratiopharm
- How to take Mirtazapine Flas Teva-ratiopharm
- Possible adverse effects
- Conservation of Mirtazapina Flas Teva-ratiopharm
- Package contents and additional information
Introduction
Leaflet: information for the user
Mirtazapine Flas Teva-ratiopharm 30 mg orodispersible tablets EFG
mirtazapine
Read the entire leaflet carefully before starting to take the medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet:
- What is Mirtazapine Flas Teva-ratiopharm and what is it used for
- What you need to know before taking Mirtazapine Flas Teva-ratiopharm
- How to take Mirtazapine Flas Teva-ratiopharm
- Possible side effects
- Storage of Mirtazapine Flas Teva-ratiopharm
- Packaging contents and additional information
1. What is Mirtazapine Flas Teva-ratiopharm and what is it used for
Mirtazapine Flas Teva-ratiopharm belongs to the group of medicines called antidepressants.
Mirtazapine Flas Teva-ratiopharm is used to treat depression in adults.
It may take 1 to 2 weeks before Mirtazapine Flas Teva-ratiopharm starts to work. After 2 to 4 weeks, you may start to feel better. You should consult your doctor if you get worse or if you do not improve after 2 to 4 weeks.
For more information, see section 3 "When can you expect to feel better".
2. What you need to know before taking Mirtazapine Flas Teva-ratiopharm
Do not take Mirtazapine Flas Teva-ratiopharm:
- If you are allergicto mirtazapine or to any of the other ingredients of this medicine (listed in section 6). In this case, consult your doctor as soon as possible before taking Mirtazapine Flas Teva-ratiopharm.
- If you are taking or have recently taken (in the last two weeks) medicines called monoamine oxidase inhibitors (MAOIs).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Mirtazapine Flas Teva-ratiopharm.
DO NOT TAKE - OR CONSULT YOUR DOCTOR BEFORE STARTING TO TAKE mirtazapine:
If you have ever suffered from a severe skin rash or skin peeling, blisters, or sores in the mouthafter taking mirtazapine or other medicines.
Children and adolescents
Mirtazapine Flas Teva-ratiopharm should not normally be used in the treatment of children and adolescents under 18 years of age, as efficacy has not been established. At the same time, you should know that in patients under 18 years of age, there is a greater risk of adverse effects such as suicidal attempts, suicidal ideation, and hostility (mainly aggression, confrontational behavior, and irritation) when taking this class of medicines. Nevertheless, the doctor may prescribe Mirtazapine Flas Teva-ratiopharm to patients under 18 years of age when he decides that it is most convenient for the patient. If the doctor has prescribed mirtazapine to a patient under 18 years of age and you wish to discuss this decision, please return to your doctor. You should inform your doctor if any of the symptoms mentioned above appear or worsen in patients under 18 years of age who are taking Mirtazapine Flas Teva-ratiopharm. Additionally, the long-term safety effects related to growth, maturity, and development of knowledge and behavior of Mirtazapine Flas Teva-ratiopharm in this age group are not yet known. Additionally, a significant weight gain has been observed more frequently in patients in this age group treated with mirtazapine, compared to adults treated.
Suicidal ideas and worsening of depression
If you are depressed, you may sometimes have ideas of harming yourself or committing suicide.
This could get worse when you start taking antidepressants for the first time, as these medicines usually take two weeks or sometimes more to work.
You may be more likely to think this way if:
- if you have previously had suicidal thoughts or thoughts of harming yourself.
- if you are a young adult. Clinical trial information has shown an increased risk of suicidal behavior in adults under 25 years of age with psychiatric disorders and who are being treated with an antidepressant.
If you have thoughts of harming yourself or committing suicide at any time, consult your doctor or go to a hospital immediately.
It may be helpful to tell a close relative or friendthat you are depressed and ask them to read this leaflet. You can ask them to tell you if they think your depression is getting worse, or if they are concerned about changes in your behavior.
Also, be especially careful with Mirtazapine Flas Teva-ratiopharm
- If you have or have ever had any of the following disorders.
If you have not already done so, inform your doctor about these situations before taking Mirtazapine Flas Teva-ratiopharm:
- seizures(epilepsy). If seizures appear or your seizures become more frequent, stop taking Mirtazapine Flas Teva-ratiopharm and contact your doctor immediately;
- liver disease, including jaundice. If jaundice appears, stop taking Mirtazapine Flas Teva-ratiopharm and contact your doctor immediately;
- kidney disease;
- heart diseaseor low blood pressure;
- schizophrenia.If psychotic symptoms, such as paranoid thoughts, become more frequent or severe, contact your doctor immediately;
- bipolar depression(alternating periods of excitement/hyperactivity and periods of depression). If you start to feel excited or overexcited, stop taking Mirtazapine Flas Teva-ratiopharm and contact your doctor immediately;
- diabetes(you may need to adjust your insulin or other antidiabetic medication dose);
- eye diseases, such as increased pressure in the eye (glaucoma);
- difficulty urinating, which could be due to an enlarged prostate;
- certain types of heart diseasethat can change your heart rate, a recent heart attack, heart failure, or taking certain medications that can affect your heart rate.
- If signs of infection appear, such as unexplained high fever, sore throat, and sores in the mouth.
If you stop taking Mirtazapine Flas Teva-ratiopharm and contact your doctor immediately to have a blood test.
In rare cases, these symptoms can be signs of changes in blood cell production in the bone marrow. Although rare, these symptoms usually appear 4-6 weeks after treatment.
- If you are an elderly person. You may be more sensitive to the adverse effects of antidepressant medicines.
Severe skin reactions have been reported with the use of mirtazapine, such as Stevens-Johnson syndrome (SSJ), toxic epidermal necrolysis (NET), and drug reaction with eosinophilia and systemic symptoms (DRESS). Discontinue use and seek medical attention immediately if you notice any of the symptoms described in section 4 related to these severe skin reactions.
If you have ever suffered from severe skin reactions, do not restart treatment with mirtazapine.
Taking Mirtazapine Flas Teva-ratiopharm with other medicines
Tell your doctor or pharmacist that you are taking/using, have recently taken/used, or may need to take/use any other medicine.
Do not take Mirtazapine Flas Teva-ratiopharmwith:
- monoamine oxidase inhibitors(MAOIs). Also, do not take Mirtazapine Flas Teva-ratiopharm during the two weeks after stopping MAOIs. If you stop taking mirtazapine, do not take MAOIs during the following two weeks. Examples of MAOIs are moclobemide, tranylcypromine (both are antidepressants), and selegiline (for Parkinson's disease).
Be carefulif you take Mirtazapine Flas Teva-ratiopharm with:
- antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), venlafaxine, and L-tryptophan or triptans(used for migraine), tramadol(for pain), linezolid(an antibiotic), lithium(used to treat some psychiatric disorders), methylene blue(used to treat high levels of methemoglobin in the blood), and St. John's Wort preparations -Hypericum perforatum(herbal medicine for depression). In very rare cases, Mirtazapine Flas Teva-ratiopharm alone or with these medicines may cause a condition called serotonin syndrome. Some symptoms of this syndrome are: unexplained fever, sweating, palpitations, diarrhea, muscle contractions (involuntary), shivering, exaggerated reflexes, agitation, mood changes, and loss of consciousness. If you experience a combination of these symptoms, consult your doctor immediately.
- the antidepressant nefazodone. It may increase the amount of mirtazapine in the blood. Inform your doctor if you are taking this medicine. It may be necessary to decrease the dose of mirtazapine or increase it again when stopping nefazodone.
- medicines for anxiety or insomniasuch as benzodiazepines.
- medicines for schizophreniasuch as olanzapine.
- medicines for allergiessuch as cetirizine.
- medicines for severe painsuch as morphine.
In combination with these medicines, mirtazapine may increase the drowsiness caused by these medicines.
- infection medicines:medicines for bacterial infections (such as erythromycin), medicines for fungal infections (such as ketoconazole), and medicines for HIV/AIDS (HIV protease inhibitors), and medicines for stomach ulcers(such as cimetidine).
If taken with mirtazapine, these medicines may increase the amount of mirtazapine in the blood. Inform your doctor if you are taking these medicines. It may be necessary to decrease the dose of mirtazapine or increase it again when stopping these medicines.
- medicines for epilepsysuch as carbamazepine and phenytoin;
- medicines for tuberculosissuch as rifampicin.
If taken with mirtazapine, these medicines may decrease the amount of mirtazapine in the blood. Inform your doctor if you are taking these medicines. It may be necessary to increase the dose of mirtazapine or decrease it again when stopping these medicines.
- medicines to prevent blood clottingsuch as warfarin.
Mirtazapine may increase the effects of warfarin in the blood. Inform your doctor if you are taking this medicine. In case of taking them together, it is recommended that the doctor perform blood tests.
- medicines that can affect heart rhythm,such as certain antibiotics and some antipsychotics.
Taking Mirtazapine Flas Teva-ratiopharm with food and alcohol
You may feel drowsy if you drink alcohol while being treated with Mirtazapine Flas Teva-ratiopharm.
It is recommended not to drink any alcohol.
You can take mirtazapine with or without food.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Limited experience with the administration of mirtazapine to pregnant women does not indicate an increased risk. However, caution should be exercised if used during pregnancy.
If you use mirtazapine until or shortly before delivery, your baby will be examined for possible adverse effects.
When similar medicines (called serotonin reuptake inhibitors: SSRIs) are taken during pregnancy, they may increase the risk of the baby suffering from a serious disease called persistent pulmonary hypertension of the newborn (PPHN), causing the baby to breathe faster and appear blue. These symptoms usually start within the first 24 hours after birth. If this happens in your case, you should contact your midwife and/or doctor immediately.
Breastfeeding
Consult your doctor if you can breastfeed while taking Mirtazapine Flas Teva-ratiopharm.
Driving and using machines
Mirtazapine Flas Teva-ratiopharm may affect your concentration or alertness. Make sure your abilities are not affected before driving or using machinery. If your doctor has prescribed Mirtazapine Flas Teva-ratiopharm to a patient under 18 years of age, make sure that concentration and alertness are not affected before circulating (e.g., by bicycle).
Mirtazapine Flas Teva-ratiopharm orodispersible tablets contain aspartame, a source of phenylalanine.
This medicine may be harmful to people with phenylketonuria because it contains aspartame, which is a source of phenylalanine.
3. How to take Mirtazapine Flas Teva-ratiopharm
Follow the instructions for administration of this medicine indicated by your doctor or pharmacist. Consult your doctor or pharmacist if you have any doubts.
How much to take
The recommended initial dose is 15 or 30 mg per day. Your doctor may recommend increasing the dose after a few days to the amount that is best for you (between 15 and 45 mg per day). The dose is usually the same for all ages. However, if you are an elderly person or if you have kidney or liver disease, your doctor may change the dose.
When to take it
Take mirtazapine at the same time every day.
It is best to take the dose of Mirtazapine Flas Teva-ratiopharm once before bedtime. However, your doctor may recommend that you divide your dose of mirtazapine in the morning and before bedtime.
The highest dose should be taken before bedtime.
Take the orodispersible tablet as follows
The tablets are taken orally.
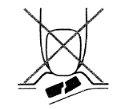
- Do not press the orodispersible tablet
To avoid crushing the orodispersible tablet, do not press the blister (Figure A)
Fig. A.
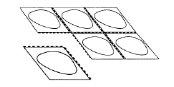
- Separate a blister
Each blister is separated by perforations. Separate a blister following the perforated lines (Figure 1)
Fig. 1
- Open the blister
Carefully remove the lamination, starting from the corner where the aluminum cover is not sealed (Figures 2 and 3)
Fig. 2
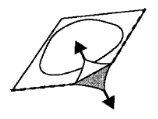
Fig. 3
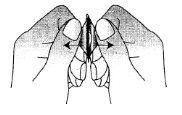
- Remove the orodispersible tablet
Remove the orodispersible tablet with dry hands and place it on the tongue (Figure 4).
Fig. 4
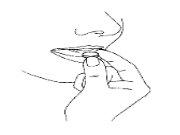
It will dissolve quickly and can be swallowed without water.
When can you expect to feel better
Normally, Mirtazapine Flas Teva-ratiopharm will start to work after 1 or 2 weeks, and after 2 to 4 weeks, you may start to feel better.
It is essential to discuss the effects of mirtazapine with your doctor during the first few weeks of treatment.
Between 2 and 4 weeks after starting to take mirtazapine, discuss with your doctor how this medicine has affected you.
If you still do not feel better, your doctor may prescribe a higher dose. In that case, discuss again with your doctor after another 2-4 weeks.
Normally, you will need to take Mirtazapine Flas Teva-ratiopharm until the symptoms of depression have disappeared for 4-6 months.
If you take more Mirtazapine Flas Teva-ratiopharm than you should:
If you or someone else takes too much Mirtazapine Flas Teva-ratiopharm, consult a doctor or pharmacist immediately. You can also call the Toxicology Information Service, phone: 91 562 04 20.
The most likely symptoms of an overdose of Mirtazapine Flas Teva-ratiopharm (without other medicines or alcohol) are drowsiness, disorientation, and palpitations.The symptoms of a possible overdose may include changes in your heart rate (fast, irregular heartbeat) and/or fainting, which could be symptoms of a potentially life-threatening condition known as Torsades de pointes.
If you forgot to take Mirtazapina Flas Teva-ratiopharm:
If you have to take your dose once a day:
- do not take a double dose to make up for missed doses. Take the next dose at the usual time.
If you have to take your dose twice a day:
If you interrupt treatment with Mirtazapina Flas Teva-ratiopharm
Stop taking Mirtazapina Flas Teva-ratiopharm only if you consult your doctor.
If you stop too soon, depression may recur. When you feel better, talk to your doctor. Your doctor will decide when you can stop treatment.
Do not stop taking Mirtazapina Flas Teva-ratiopharm abruptly, even if depression has disappeared. If you stop taking mirtazapine abruptly, you may feel sick, dizzy, agitated, or anxious and have a headache. These symptoms can be avoided by gradually stopping treatment. Your doctor will indicate how to gradually reduce the dose.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you experience any of the following serious adverse effects, stop taking mirtazapine and inform your doctor immediately.
Uncommon(may affect up to 1 in 100 people)
- Feeling of exaggerated euphoria (mania)
Rare(may affect up to 1 in 1,000 people):
- Yellowing of the eyes or skin; may suggest liver function alterations (jaundice)
Frequency not known(frequency cannot be estimated from available data):
- Signs of infection such as high, unexplained, and sudden fever, sore throat, and mouth ulcers (agranulocytosis)
In rare cases, mirtazapine may cause alterations in blood cell production (bone marrow depression). Some people become less resistant to infections because mirtazapine can cause a temporary decrease in white blood cells (granulocytopenia). In rare cases, mirtazapine can also cause a decrease in red and white blood cells and platelets (aplastic anemia), a decrease in platelets (thrombocytopenia), or an increase in the number of white blood cells in the blood (eosinophilia).
- Seizure (convulsions)
- Combination of symptoms such as unexplained fever, sweating, palpitations, diarrhea, muscle contractions (involuntary), exaggerated reflexes, agitation, mood changes, loss of consciousness, and increased saliva production. In very rare cases, these symptoms can be signs of a disorder called "serotonin syndrome"
- Thoughts of self-harm or suicide
- Red patches on the torso, such as circumscribed or circular macules, often with blisters in the center, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin erythemas can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis).
- Generalized erythema, elevated body temperature, and enlarged lymph nodes (DRESS or drug hypersensitivity syndrome).
Other possible adverse effects with mirtazapine are:
Very common (may affect more than 1 in 10 people):
- Increased appetite and weight gain
- Somnolence
- Headache
- Dry mouth
Common (may affect up to 1 in 10 people):
- Lethargy
- Dizziness
- Tremor
- Nausea
- Diarrhea
- Vomiting
- Constipation
- Urticaria or skin rash (exanthema)
- Joint pain (arthralgia) or muscle pain (myalgia)
- Back pain
- Dizziness or fainting when standing up quickly (orthostatic hypotension)
- Swelling (usually in ankles or feet) due to fluid retention (edema)
- Fatigue
- Vivid dreams
- Confusion
- Anxiety
- Difficulty sleeping
- Memory problems, which in most cases were resolved when treatment was discontinued
Uncommon (may affect up to 1 in 100 people):
- Strange sensation in the skin, such as burning, pricking, tingling, or numbness (paresthesia)
- Involuntary movements of agitation of the legs during sleep
- Fainting (syncope)
- Numbness of the mouth (oral hypoesthesia)
- Low tension
- Nightmares
- Agitation
- Hallucinations
- Inability to remain still
Rare (may affect up to 1 in 1,000 people):
- Tics or muscle contractions (myoclonus)
- Aggression
- Abdominal pain, nausea; this may indicate pancreas inflammation (pancreatitis)
Frequency not known(frequency cannot be estimated from available data):
- Abnormal sensations in the mouth (oral paresthesia)
- Swelling in the mouth (oral edema)
- Generalized swelling
- Localized swelling
- Hyponatremia
- Inadequate secretion of antidiuretic hormone.
- Severe skin reactions (bullous dermatitis, erythema multiforme)
- Sleepwalking (somnambulism)
- Speech problems
- Increased levels of creatine kinase in the blood
- Difficulty urinating (urinary retention)
- Muscle pain, stiffness, and/or weakness, darkening or discoloration of urine (rhabdomyolysis)
- Increased levels of prolactin hormone in the blood (hyperprolactinemia, including symptoms of breast enlargement and/or milky discharge from the nipple).
- Painful and prolonged erection of the penis
Other adverse effects in children and adolescents
In minors under 18 years of age, the following adverse effects were frequently observed in clinical trials: significant weight gain, urticaria, and increased triglycerides in the blood.
Reporting of adverse effects:
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Mirtazapina Flas Teva-ratiopharm
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and blister. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Mirtazapina Flas Teva-ratiopharm
- The active ingredient is mirtazapine. Each orodispersible tablet contains 30 mg of mirtazapine.
- The other components are: crospovidone (type B), mannitol (E421), microcrystalline cellulose (E460), aspartame (E951), anhydrous colloidal silica, magnesium stearate (E572), strawberry flavor (artificial flavors, corn maltodextrin, triethyl citrate, and propylene glycol), peppermint flavor (artificial flavors, corn starch).
Appearance of the product and package contents
Orodispersible tablets.
Round, white tablets, marked with "37" on one side and with "A" on the other side, with a circular relief edge.
Mirtazapina Flas Teva-ratiopharm 30 mg is available in packages containing 6, 10, 18, 20, 30, 48, 50, 60, 90, 96, and 100 orodispersible tablets.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Teva Pharma, S.L.U.
C/Anabel Segura, 11 Edificio Albatros B, 1ª planta
28108 Alcobendas (Madrid) Spain
Manufacturer
Merckle GmbH
Ludwig-Merckle-Strasse 3
89143 Blaubeuren (Germany)
Or
Teva Operations Poland Sp. z o.o.
Ul. Mogilska 80
31-546 Kraków
Poland
This medicine is authorized in the EEA Member States with the following names:
Member State | Medicine name |
Germany | Mirtazapin-ratiopharm 30 mg Schmelztabletten |
Italy | Mirtazapina Teva Italia 30 mg compresse orodispersibili |
Netherlands | Mirtazapine ratiopharm dispergeerbaar 30 mg, orodispergeerbare tabletten |
Portugal | Mirtazapina ratiopharm |
Spain | Mirtazapina Flas Teva-ratiopharm 15 mg comprimidos bucodispersables EFG Mirtazapina Flas Teva-ratiopharm 30 mg comprimidos bucodispersables EFG |
Date of the last revision of this prospectus:July 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
You can access detailed and updated information about this medicine by scanning the QR code included in the packaging with your mobile phone (smartphone). You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/69704/P_69704.html
- Country of registration
- Average pharmacy price17.05 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MIRTAZAPINE TEVA-RATIOPHARM 30 mg ORALLY DISINTEGRATING TABLETSDosage form: TABLET, 15 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 30 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 15 mgActive substance: mirtazapineManufacturer: Almus Farmaceutica S.A.U.Prescription required
Online doctors for MIRTAZAPINE TEVA-RATIOPHARM 30 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about MIRTAZAPINE TEVA-RATIOPHARM 30 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











