
LEVETIRACETAM TARBIS FARMA 100 MG/ML ORAL SOLUTION

How to use LEVETIRACETAM TARBIS FARMA 100 MG/ML ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Levetiracetam Tarbis Farma 100 mg/ml Oral Solution EFG
levetiracetam
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Levetiracetam Tarbis Farma and what is it used for
- What you need to know before taking Levetiracetam Tarbis Farma
- How to take Levetiracetam Tarbis Farma
- Possible side effects
- Storage of Levetiracetam Tarbis Farma
- Package Contents and Additional Information
1. What is Levetiracetam Tarbis Farma and what is it used for
Levetiracetam is an antiepileptic medication (a medication for the treatment of seizures in epilepsy).
This medication is used:
- alone in adults and adolescents 16 years of age or older with newly diagnosed epilepsy to treat a form of epilepsy. Epilepsy is a disease where patients have seizures. Levetiracetam is used for the form of epilepsy in which seizures initially affect only one side of the brain but may later spread to larger areas on both sides of the brain (partial onset seizures with or without secondary generalization). Your doctor has prescribed levetiracetam to reduce the number of seizures.
- in combination with other antiepileptic medications to treat:
- partial onset seizures with or without secondary generalization in adults, adolescents, children, and infants from 1 month of age.
- myoclonic seizures (short, shock-like muscle jerks) in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy.
- primary generalized tonic-clonic seizures (major seizures, including loss of consciousness) in adults and adolescents from 12 years of age with idiopathic generalized epilepsy (a type of epilepsy thought to have a genetic cause).
2. What you need to know before taking Levetiracetam Tarbis Farma
Do not take Levetiracetam Tarbis Farma
- If you are allergic to levetiracetam, pyrrolidone derivatives, or any of the other components of this medication (listed in section 6).
Warnings and Precautions
Consult your doctor before starting to take this medication
- If you have kidney problems, follow your doctor's instructions, who will decide if you need to adjust the dose to take.
- If you notice any decrease in your child's growth or unexpected pubertal development, contact your doctor.
- A small number of people taking antiepileptics such as levetiracetam have had thoughts of self-harm or suicide. If you have any symptoms of depression and/or suicidal thoughts, contact your doctor.
- If you have a medical history or family history of irregular heart rhythm (visible on an electrocardiogram), or if you have a disease and/or are taking a treatment that makes you prone to cardiac arrhythmias or electrolyte imbalances.
Tell your doctor or pharmacist if any of the following side effects worsen or last more than a few days:
- Abnormal thoughts, feeling irritable, or reacting more aggressively than usual, or if you or your family and friends notice significant changes in your mood or behavior.
- Worsening of epilepsy
In rare cases, epileptic seizures may worsen or occur more frequently, mainly during the first month after starting treatment or increasing the dose. If you experience any of these new symptoms while taking this medication, consult a doctor as soon as possible.
If you experience any of these new symptoms while taking this medication, consult a doctor as soon as possible.
Children and Adolescents
- Monotherapy with Levetiracetam Tarbis Farma is not indicated in children and adolescents under 16 years of age.
Other Medications and Levetiracetam Tarbis Farma
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Do not take macrogol (a medication used as a laxative) within one hour before and one hour after taking levetiracetam, as it may reduce its effect.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medication. Levetiracetam should only be used during pregnancy if, after careful evaluation, your doctor considers it necessary.
Do not stop your treatment without discussing it with your doctor first.
The risk of birth defects for the baby cannot be completely ruled out.
Breastfeeding is not recommended during treatment.
Driving and Using Machines
Levetiracetam may affect your ability to drive or operate tools or machinery, as it may cause drowsiness. This is more likely at the start of treatment or when the dose is increased. You should not drive or use machinery until it is verified that your ability to perform these activities is not affected.
Levetiracetam Tarbis Farma contains methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), and maltitol (E965).
Methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) may cause allergic reactions.
Maltitol: If your doctor tells you that you have an intolerance to some sugars, consult your doctor before taking this medication.
This medication contains less than 23 mg of sodium (1 mmol) per ml; it is essentially "sodium-free".
3. How to Take Levetiracetam Tarbis Farma
Follow the administration instructions of this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Levetiracetam should be taken twice a day, once in the morning and once at night, approximately at the same time each day.
Take the oral solution according to your doctor's instructions.
Monotherapy (from 16 years of age)
Adults (≥18 years) and adolescents (from 16 years of age):
For patients from 4 years of age, measure the correct dose using the 10 ml syringe included in the box.
Recommended dose: levetiracetam is taken twice a day, in two equal doses, each individual dose between 5 ml (500 mg) and 15 ml (1500 mg).
When starting to take Levetiracetam, your doctor will prescribe a lower dose for two weeks before administering the lowest daily dose.
Concomitant Therapy
Dose in adults and adolescents (from 12 to 17 years):
For patients from 4 years of age, measure the correct dose using the 10 ml syringe included in the box.
Recommended dose: Levetiracetam is taken twice a day, in two equal doses, each individual dose between 5 ml (500 mg) and 15 ml (1500 mg).
Dose in children from 6 months of age:
Your doctor will prescribe the most suitable pharmaceutical form of levetiracetam according to age, weight, and dose.
For children from 6 months to 4 years of age, measure the correct dose using the 3 ml syringe included in the box.
For children over 4 years of age, measure the correct dose using the 10 ml syringe included in the box.
Recommended dose: Levetiracetam is taken twice a day, in two equal doses, each individual dose between 0.1 ml (10 mg) and 0.3 ml (30 mg) per kilogram of the child's body weight (see the following table for dose examples).
Dose in children from 6 months of age:
Weight | Initial dose: 0.1 ml/kg twice a day | Maximum dose: 0.3 ml/kg twice a day |
6 kg | 0.6 ml twice a day | 1.8 ml twice a day |
8 kg | 0.8 ml twice a day | 2.4 ml twice a day |
10 kg | 1 ml twice a day | 3 ml twice a day |
15 kg | 1.5 ml twice a day | 4.5 ml twice a day |
20 kg | 2 ml twice a day | 6 ml twice a day |
25 kg | 2.5 ml twice a day | 7.5 ml twice a day |
From 50 kg | 5 ml twice a day | 15 ml twice a day |
Dosing in Infants (from 1 month to less than 6 months):
For infants from 1 month to less than 6 months of age, measure the correct dose using the 1 ml syringe included in the box.
Recommended dose: Levetiracetam is taken twice a day, in two equal doses, each individual dose between 0.07 ml (7 mg) and 0.21 ml (21 mg) per kilogram of the infant's body weight (see the following table for dose examples).
Dose in Infants (from 1 month to less than 6 months of age):
Weight | Initial dose: 0.07 ml/kg twice a day | Maximum dose: 0.21 ml/kg twice a day |
4 kg | 0.3 ml twice a day | 0.85 ml twice a day |
5 kg | 0.35 ml twice a day | 1.05 ml twice a day |
6 kg | 0.45 ml twice a day | 1.25 ml twice a day |
7 kg | 0.5 ml twice a day | 1.5 ml twice a day |
Method of Administration:
This medication is for oral use. After measuring the correct dose with the suitable syringe, Levetiracetam oral solution can be diluted in a glass of water or in a baby bottle. You can take Levetiracetam with or without food. After oral administration of levetiracetam, its bitter taste may be noticed.
Instructions on how to use the syringe:
- Open the vial: press the cap and unscrew it counterclockwise (figure 1)
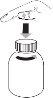
- Follow these steps the first time you take Levetiracetam:
- Remove the adapter from the oral syringe (figure 2).
- Insert the adapter into the top of the vial (figure 3). Make sure it is properly placed. It is not necessary to remove the adapter after use.
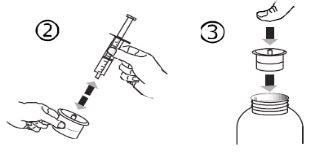
- Follow these steps each time you take Levetiracetam
- Insert the oral syringe into the adapter opening (figure 4).
- Turn the vial upside down (figure 5).
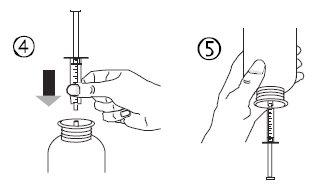
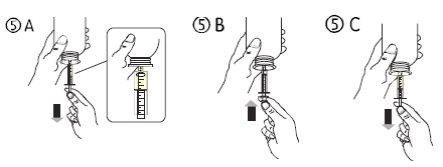
- Hold the vial upside down with one hand and with the other hand fill the oral syringe.
- Pull the plunger down to fill the oral syringe with a small amount of solution (figure 5A).
- Then, push the plunger up to eliminate any air bubbles (figure 5B).
- Pull the plunger down to the dose mark in milliliters (ml) indicated on the oral syringe and prescribed by your doctor (figure 5C). The plunger may retract in the cylinder at the first dose, so you must ensure that you keep the plunger in its position until you disconnect the syringe from the vial.
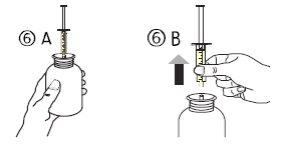
- Put the vial upright (figure 6A). Remove the syringe from the adapter (figure 6B).
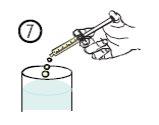
- Empty the contents of the syringe into a glass of water or a baby bottle, pushing the plunger down to the end of the syringe (figure 7).
- Drink the entire contents of the glass or baby bottle.
- Close the vial with the plastic screw cap (it is not necessary to remove the adapter).
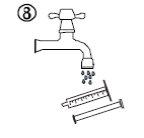
- To clean the syringe, rinse it only with cold water, moving the plunger several times up and down to absorb and expel the water, without separating the two components (figure 8).
- Store the vial, oral syringe, and package leaflet in the box.
Duration of treatment:
- Levetiracetam is used as a chronic treatment. You should continue treatment with levetiracetam for the time indicated by your doctor.
- Do not stop your treatment without your doctor's recommendation, as your seizures may increase.
If you take more Levetiracetam Tarbis Farma than you should
The possible side effects of an overdose of levetiracetam are drowsiness, agitation, aggression, decreased alertness, respiratory inhibition, and coma.
Contact your doctor if you have taken more oral solution than you should. Your doctor will establish the best possible treatment for the overdose.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone 915 620 420, indicating the medication and the amount used.
If you forget to take Levetiracetam Tarbis Farma:
Contact your doctor if you have missed one or more doses.
Do not take a double dose to make up for missed doses.
If you stop treatment with Levetiracetam Tarbis Farma:
The end of treatment with levetiracetam should be done gradually to avoid an increase in seizures. If your doctor decides to stop your treatment with levetiracetam, he/she will give you instructions for the gradual withdrawal of levetiracetam.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, levetiracetam can cause adverse effects, although not all people suffer from them.
Inform your doctor immediately, or go to the emergency department of your nearest hospital if you experience:
- weakness, dizziness, or difficulty breathing, as these may be signs of a severe allergic reaction (anaphylaxis)
- swelling of the face, lips, tongue, or throat (Quincke's edema)
- flu-like symptoms and rash on the face followed by a prolonged rash with high temperature, elevated liver enzyme levels in blood tests, and an increase in a type of white blood cells (eosinophilia) and enlarged lymph nodes, and the involvement of other body organs (Drug Hypersensitivity Reaction with Eosinophilia and Systemic Symptoms (DRESS))
- symptoms such as low urine volume, fatigue, nausea, vomiting, confusion, and swelling of legs, arms, or feet, as these may be signs of sudden renal function decrease
- a skin rash that can form blisters and may appear as small targets (dark central spots surrounded by a lighter area, with a dark ring around the edge) (erythema multiforme)
- a widespread rash with blisters and skin peeling, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome)
- a more severe form that causes skin peeling on more than 30% of the body surface (toxic epidermal necrolysis)
- signs of severe mental changes or if someone around you notices signs of confusion, drowsiness (drowsiness), amnesia (memory loss), memory impairment (forgetfulness), abnormal behavior, or other neurological signs, including involuntary or uncontrolled movements. These may be symptoms of encephalopathy.
The most frequently reported adverse effects are nasopharyngitis, drowsiness (feeling of sleep), headache, fatigue, and dizziness. Adverse effects such as drowsiness, weakness, and dizziness may be more frequent when treatment is initiated or the dose is increased. However, these adverse effects should decrease over time.
Very common:may affect more than 1 in 10 people
- nasopharyngitis;
- drowsiness (feeling of sleep), headache.
Common:may affect up to 1 in 10 people
- anorexia (loss of appetite);
- depression, hostility, or aggression, anxiety, insomnia, nervousness, or irritability;
- seizures, balance disorder, dizziness (feeling of instability), lethargy (lack of energy and enthusiasm), tremor (involuntary tremor);
- vertigo (feeling of rotation);
- cough;
- abdominal pain, diarrhea, dyspepsia (heavy digestion, heartburn, and acidity), vomiting, nausea;
- skin rash;
- asthenia/fatigue (feeling of weakness).
Uncommon:may affect up to 1 in 100 people
- decrease in platelet count, decrease in white blood cells;
- weight loss, weight gain;
- suicidal thoughts and attempts, mental disorders, abnormal behavior, hallucinations, anger, confusion, panic attack, emotional instability/mood changes, agitation;
- amnesia (memory loss), memory impairment (lack of memory), abnormal coordination/ataxia (altered movement coordination), paresthesia (tingling), attention disorders (loss of concentration);
- diplopia (double vision), blurred vision;
- elevated/abnormal liver function test values;
- hair loss, eczema, itching;
- muscle weakness, myalgia (muscle pain);
- injury.
Rare:may affect up to 1 in 1,000 people
- infection;
- decrease in all types of blood cells;
- severe allergic reactions (DRESS, anaphylactic reaction (severe and important allergic reaction), Quincke's edema (swelling of face, lips, tongue, and throat));
- decrease in sodium concentration in blood;
- suicide, personality disorders (behavioral problems), abnormal thinking (slow thinking, difficulty concentrating);
- delirium;
- encephalopathy (see subsection "Inform your doctor immediately" for a detailed description of symptoms);
- epileptic seizures may worsen or occur more frequently;
- uncontrolled muscle spasms affecting the head, torso, and limbs, difficulty controlling movements, hyperkinesia (hyperactivity);
- change in heart rhythm (electrocardiogram);
- pancreatitis (inflammation of the pancreas);
- liver failure, hepatitis (inflammation of the liver);
- sudden decrease in renal function;
- skin rash, which may lead to blisters that may appear as small targets (dark central spots surrounded by a lighter area, with a dark ring around the edge) (erythema multiforme), a widespread rash with blisters and skin peeling, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome), and a more severe form that causes skin peeling on more than 30% of the body surface (toxic epidermal necrolysis);
- rhabdomyolysis (muscle tissue breakdown) and increased creatine phosphokinase in blood. The prevalence is significantly higher in Japanese patients compared to non-Japanese patients;
- limping or difficulty walking.
- combination of fever, muscle stiffness, unstable blood pressure and heart rate, confusion, low level of consciousness (may be signs of a disorder called malignant neuroleptic syndrome). The prevalence is significantly higher in Japanese patients compared to non-Japanese patients.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Medicines Monitoring System website: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Levetiracetam Tarbis Farma
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the carton after CAD. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Levetiracetam Tarbis Farma
The active ingredient is levetiracetam.
Each ml contains 100 mg of levetiracetam.
The other components are: liquid maltitol (E965), glycerol (E422), methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), sodium citrate monohydrate, citric acid (dihydrate), ammonium glycyrrhizate, potassium acesulfame (E950), grape flavor, purified water
Appearance of the Product and Package Contents
Levetiracetam Tarbis Farma 100 mg/ml oral solution EFG is a clear and colorless liquid.
Package sizes:
Ambber glass bottle of 300 ml (type III) (containing 300 ml of oral solution) with a white child-resistant cap (polyethylene), accompanied by a 10 ml graduated oral syringe (polypropylene) and a syringe adapter (polyethylene), all contained in a cardboard box.
Ambber glass bottle of 200 ml (type III) (containing 150 ml of oral solution) with a white child-resistant cap (polyethylene), accompanied by a 3 ml graduated oral syringe (polypropylene) and a syringe adapter (polyethylene), all contained in a cardboard box.
Ambber glass bottle of 200 ml (type III) (containing 150 ml of oral solution) with a white child-resistant cap (polyethylene), accompanied by a 1 ml graduated oral syringe (polypropylene) and a syringe adapter (polyethylene), all contained in a cardboard box.
Not all package sizes may be marketed.
Marketing Authorization Holder
Tarbis Farma S.L.
Gran Vía Carlos III, 94
08028 Barcelona
Spain
Manufacturer
Amarox Pharma B.V.
Rouboslaan 32
Voorschoten, 2252TR
Netherlands
This medicine is authorized in the Member States of the European Economic Area under the following names:
Netherlands: Levetiracetam Amarox 100 mg/ml drank
Germany: Levetiracetam Amarox 100 mg/ml Lösung zum Einnehmen
Spain: Levetiracetam Tarbis Farma 100 mg/ml oral solution EFG
Date of the last revision of this prospectus:March 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price28.32 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEVETIRACETAM TARBIS FARMA 100 MG/ML ORAL SOLUTIONDosage form: INJECTABLE PERFUSION, 100 mgActive substance: levetiracetamManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE PERFUSION, 100 mg/mlActive substance: levetiracetamManufacturer: Ucb PharmaPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 100 mgActive substance: levetiracetamManufacturer: Ucb PharmaPrescription required
Online doctors for LEVETIRACETAM TARBIS FARMA 100 MG/ML ORAL SOLUTION
Discuss questions about LEVETIRACETAM TARBIS FARMA 100 MG/ML ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








