
LANSOPRAZOL FLAS VIATRIS 15 mg ORALLY DISINTEGRATING TABLETS

How to use LANSOPRAZOL FLAS VIATRIS 15 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Lansoprazole Flas Viatris 15 mg Orodispersible Tablets EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Lansoprazole Flas Viatris and what is it used for
- What you need to know before you take Lansoprazole Flas Viatris
- How to take Lansoprazole Flas Viatris
- Possible side effects
- Storage of Lansoprazole Flas Viatris
- Contents of the pack and other information
1. What is Lansoprazole Flas Viatris and what is it used for
The active substance of Lansoprazole Flas Viatris is lansoprazole, which is a proton pump inhibitor.
Proton pump inhibitors reduce the amount of acid produced by the stomach.
Your doctor may prescribe you lansoprazole for the following:
- Treatment of duodenal (intestinal) and gastric ulcers.
- Treatment of inflammation of the esophagus (reflux esophagitis).
- Prevention of reflux esophagitis.
- Treatment of heartburn and acid regurgitation.
- Treatment of infections caused by the bacterium Helicobacter pylori, if administered together with an antibiotic.
- Treatment or prevention of duodenal (intestinal) or gastric ulcers in patients who require continuous treatment with non-steroidal anti-inflammatory drugs (NSAIDs) (treatment with NSAIDs is used to reduce pain or inflammation).
- Treatment of Zollinger-Ellison syndrome.
It may be that your doctor has prescribed lansoprazole to treat another disease or with a different dose than indicated in this leaflet. Follow your doctor's instructions regarding the administration of the medicine.
2. What you need to know before you take Lansoprazole Flas Viatris
Do not take Lansoprazole Flas Viatris:
- If you are allergic to lansoprazole or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Lansoprazole Flas Viatris:
- If you have liver problems. Your doctor may adjust the dose.
- If you have ever had an allergic reaction on the skin after treatment with a medicine similar to Lansoprazole Flas Viatris to reduce stomach acidity.
- If you are scheduled to undergo a specific blood test (Chromogranin A).
- If you have low levels of vitamin B12 or risk factors for it and receive treatment with these medicines for a long time. Like all medicines that reduce the amount of acid, lansoprazole may reduce the absorption of vitamin B12.
This medicine may affect the way your body absorbs vitamin B12, particularly if you need to take it for a long time. Please contact your doctor if you notice any of the following symptoms, as they may indicate low levels of vitamin B12:
- Extreme fatigue or lack of energy.
- Pins and needles.
- Red or sore tongue, mouth ulcers.
- Muscle weakness.
- Altered vision.
- Memory problems, confusion, depression.
If you experience a skin rash, especially in areas of the skin exposed to the sun, consult your doctor as soon as possible, as it may be necessary to interrupt treatment with lansoprazole. Remember to mention any other symptoms you may notice, such as joint pain.
If your doctor has prescribed Lansoprazole Flas Viatris in addition to other medicines intended to treat the infection caused by the bacterium Helicobacter pylori(antibiotics) or together with anti-inflammatory medicines to treat pain or rheumatism, also read carefully the package leaflets of these medicines.
Your doctor may have performed or may perform a complementary test called endoscopy to diagnose your disease and/or rule out a malignant disease.
When taking lansoprazole, kidney inflammation may occur. The signs and symptoms may include decreased urine volume or the presence of blood in the urine and/or hypersensitivity reactions such as fever, skin rash, and joint stiffness. You should inform your doctor of these signs.
If you take lansoprazole for more than three months, your magnesium levels in the blood may become very low. If you become tired, disoriented, dizzy, or have muscle spasms, convulsions (seizures), or an increased heart rate, contact your doctor immediately, as you may have low magnesium levels in the blood (see section 4 "Possible side effects"). A decrease in magnesium levels in the blood can lead to a decrease in calcium and potassium levels in the blood. Therefore, your doctor may monitor your magnesium levels in the blood.
The use of a proton pump inhibitor such as lansoprazole, especially for a period longer than one year, may slightly increase the risk of hip, wrist, or spine fractures. Inform your doctor if you have osteoporosis or if you are taking corticosteroids (which may increase the risk of osteoporosis).
If you take lansoprazole for a prolonged period (more than 1 year), your doctor will probably perform periodic reviews. You should inform your doctor of any new circumstance or unusual symptom.
During treatment
If you experience diarrhea during treatment with lansoprazole, contact your doctor immediately, as lansoprazole is associated with a small increase in infectious diarrhea.
Other medicines and Lansoprazole Flas Viatris
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription.
In particular, inform your doctor if you are taking medicines that contain:
- HIV protease inhibitors such as atazanavir and nelfinavir (used to treat HIV).
- Methotrexate (used to treat autoimmune diseases and cancer).
- Ketoconazole (used to treat Cushing's syndrome - when the body produces excess cortisol).
- Itraconazole (used to treat fungal infections).
- Rifampicin (used to treat tuberculosis).
- Digoxin (used to treat heart problems).
- Warfarin (used to treat blood clots).
- Theophylline (used to treat asthma).
- Tacrolimus (used to prevent transplant rejection).
- Fluvoxamine (used to treat depression and other psychiatric disorders).
- Antacids (used to treat heartburn or acid regurgitation, such as aluminum hydroxide or magnesium carbonate).
- Sucralfate (used to heal ulcers).
- St. John's Wort or hypericum (Hypericum perforatum) (used to treat mild depression).
Pregnancy and breastfeeding
Lansoprazole is not recommended during pregnancy, as there are no data available.
It is unknown whether lansoprazole is present in breast milk. Talk to your doctor to determine whether it is more beneficial to continue taking this medicine or to continue breastfeeding your baby.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Sometimes, patients taking lansoprazole may experience side effects such as dizziness, vertigo (a feeling of rotational movement when sitting or standing), drowsiness, and vision problems. If you experience any of these side effects, you should act with caution, as your reaction ability may be reduced.
You are the only one responsible for deciding whether you are in a condition to drive vehicles or perform other activities that require a high level of concentration. Due to its effects or adverse reactions, one of the factors that can reduce your ability to perform these activities safely is the use of medicines.
In the following sections, you will find descriptions of these effects. Read the information in this leaflet carefully. If you have any doubts, consult your doctor or pharmacist.
Lansoprazole Flas Viatris contains saccharose, aspartame, and sodium
This medicine contains 5.97 mg of aspartame per tablet.
Aspartame is a source of phenylalanine. It may be harmful if you have phenylketonuria, a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly.
This medicine contains saccharose.
If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine.
This medicine contains less than 1 mmol of sodium (23 mg) per tablet, i.e., it is essentially "sodium-free".
3. How to take Lansoprazole Flas Viatris
Follow exactly the administration instructions of this medicine indicated by your doctor. If you have any doubts, consult your doctor or pharmacist.
The dose of lansoprazole depends on your general condition. The recommended doses of lansoprazole for adults are indicated below. Sometimes, your doctor may prescribe a different dose and indicate a different duration of treatment.
Treatment of heartburn and acid regurgitation:one 15 mg or 30 mg orodispersible tablet per day for 4 weeks. If symptoms persist, inform your doctor. If symptoms do not improve after 4 weeks, consult your doctor.
Treatment of duodenal (intestinal) ulcers:one 30 mg orodispersible tablet per day for 2 weeks.
Treatment of gastric ulcers:one 30 mg orodispersible tablet per day for 4 weeks.
Treatment of inflammation of the esophagus (reflux esophagitis):one 30 mg orodispersible tablet per day for 4 weeks.
Long-term prevention of reflux esophagitis:one 15 mg orodispersible tablet per day; your doctor may adjust the dose to one 30 mg orodispersible tablet per day.
Treatment of the infection caused byHelicobacter pylori:the recommended dose is one 30 mg orodispersible tablet together with two different antibiotics in the morning and one 30 mg orodispersible tablet together with two different antibiotics in the evening. Treatment is usually daily for 7 days.
The recommended antibiotic combinations are:
- 30 mg of lansoprazole with 250-500 mg of clarithromycin and 1000 mg of amoxicillin.
- 30 mg of lansoprazole with 250 mg of clarithromycin and 400-500 mg of metronidazole.
If you receive anti-infective treatment due to an ulcer, it is unlikely that the ulcer will recur if the infection is treated satisfactorily. To get the best results from your medicine, take it at the right time and do not miss any dose.
Treatment of duodenal (intestinal) or gastric ulcers in patients who require continuous treatment with non-steroidal anti-inflammatory drugs (NSAIDs):one 30 mg orodispersible tablet per day for 4 weeks.
Prevention of duodenal (intestinal) or gastric ulcers in patients who require continuous treatment with NSAIDs:one 15 mg orodispersible tablet per day; your doctor may adjust the dose to one 30 mg orodispersible tablet per day.
Zollinger-Ellison syndrome:the initial recommended dose is two 30 mg orodispersible tablets per day; subsequently, based on your response to treatment with lansoprazole, the doctor will decide the best dose for you.
Use in patients with liver problems
If you have moderate or severe liver problems, your doctor may prescribe half of the recommended dose.
Use in elderly patients
Your doctor may prescribe less than the recommended dose if you are an elderly patient. The maximum recommended dose for elderly patients is 30 mg per day.
Use in children
Lansoprazole should not be administered to children.
How to take it
To get the best results with the medicine, take lansoprazole at least 30 minutes before food intake.
If you take lansoprazole once a day, try to take it at the same time every day. You can get the best results if you take lansoprazole on an empty stomach in the morning.
If you take lansoprazole twice a day, take the first dose in the morning and the second dose in the afternoon.
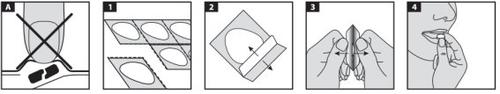
Lansoprazole tablets break easily, so you should handle them carefully. Do not handle the tablets with wet hands, as they can break.
Lansoprazole Viatris in peelable or press-through blisters.
- Only for peelable blisters, hold the blister strip by the edges and separate one blister from the rest of the strip by gently tearing the perforations around the blister.
- Carefully peel off the back. For non-perforated blisters, be careful not to peel off the back of the adjacent tablets.
- Gently push the tablet out.
- Place the tablet in your mouth. The tablet will dissolve directly in your mouth and can be swallowed more easily.
You can also swallow the tablet whole with a glass of water.
Your doctor may give you instructions to administer the tablet through a syringe, in case you have severe difficulty swallowing.
If you use an oral syringe:
- Remove the plunger from the syringe (at least a 5 ml syringe for the 15 mg tablet and a 10 ml syringe for the 30 mg tablet).
- Place the tablet in the cylinder.
- Put the plunger back in the syringe.
- For the 15 mg tablet: pull the plunger to introduce 4 ml of tap water into the syringe.
- For the 30 mg tablet: pull the plunger to introduce 10 ml of tap water into the syringe.
- Hold the syringe upside down and pull the plunger to introduce 1 ml of air into the syringe.
- Gently shake the syringe for 10-20 seconds until the tablet is dispersed.
- The contents can be emptied directly into the mouth.
- Refill the syringe with 2-5 ml of tap water to remove any remaining contents and empty it into the mouth.
- Repeat this last step if necessary.
If you use a nasogastric tube:
It is essential to carefully evaluate the suitability of the selected tube. The recommended diameter of the nasogastric tube to be used is 3.3 mm (size 10 in the French scale) or larger.
- Remove the plunger from the syringe (use at least a 25 ml syringe for the 15 mg tablet and a 50 ml syringe for the 30 mg tablet).
- Place the tablet in the cylinder.
- Put the plunger back in the syringe.
- For the 15 mg tablet: pull the plunger to introduce 10 ml of tap water into the syringe.
- For the 30 mg tablet: pull the plunger to introduce 25 ml of tap water into the syringe.
- Hold the syringe upside down and pull the plunger to introduce 5 ml of air into the syringe.
- Gently shake the syringe for 10-20 seconds until the tablet is dispersed.
- Connect the syringe to the nasogastric tube and empty the contents of the syringe into it.
- For the 15 mg tablet: refill the syringe with 10 ml of tap water and empty its contents into the tube.
- For the 30 mg tablet: refill the syringe with 25 ml of tap water and empty its contents into the tube.
If you take more Lansoprazole Flas Viatris than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take Lansoprazole Flas Viatris
If you forget to take a dose, take it as soon as possible, unless the time of the next dose is near. If this happens, do not take the forgotten dose and continue taking lansoprazole normally.
Do not take a double dose to make up for forgotten doses.
If you stop taking Lansoprazole Flas Viatris
Do not stop treatment prematurely because the symptoms have improved. It is possible that your condition has not been completely cured and may recur if you do not complete the entire treatment.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Stop taking this medicine and inform your doctor immediately or go to the nearest hospital if you think you may have any of the following adverse effects:
- Increased number of infections causing fever, severe chills, sore throat, or respiratory infections. These symptoms could be indicative of a reduction in the number of white blood cells (leukopenia - uncommon, may affect up to 1 in 100 people).
- Fatigue, coldness of hands and feet, pale skin with spontaneous bruising, bleeding that lasts longer than usual. These symptoms could be indicative of a severe reduction in all types of blood cells (pancytopenia - very rare, may affect up to 1 in 10,000 people).
- Sudden severe dull pain in the upper abdomen. The pain may spread to the back and may worsen after eating. These symptoms could be indicative of pancreas inflammation (rare, may affect up to 1 in 1,000 people).
- Yellowing of the skin or whites of the eyes, loss of appetite, discomfort (nausea), pale-colored stools, or cloudy urine. These symptoms could be indicative of severe liver problems (rare, may affect up to 1 in 1,000 people).
- Swelling of the face, lips, tongue, or throat causing difficulty swallowing or breathing, fever, rash commonly known as hives, inflammation, and sometimes a rapid decrease in blood pressure. These symptoms could be indicative of a severe allergic reaction, including anaphylactic shock (rare, may affect up to 1 in 1,000 people).
- Production of little or no urine, cloudy or bloody urine, pain while urinating, or lower back pain. These symptoms could be indicative of severe kidney problems. These symptoms may be indicative of severe kidney problems (rare, may affect up to 1 in 1,000 people).
- Redness of the skin, blistering, severe inflammation, peeling, and skin loss. These symptoms could be indicative of severe skin reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS) (frequency not known, cannot be estimated from available data), Stevens-Johnson syndrome, or toxic epidermal necrolysis (very rare, may affect up to 1 in 10,000 people).
- Severe or persistent diarrhea. If you experience this adverse effect, inform your doctor, as this medicine has been associated with a slight increase in infectious diarrhea (very rare, may affect up to 1 in 10,000 people).
- Skin rash, possibly with joint pain (frequency not known, cannot be estimated from available data).
Other possible adverse effects:
Common(may affect up to 1 in 10 people):
- Headache, dizziness.
- Diarrhea, constipation, stomach pain, discomfort (nausea) or vomiting, flatulence, dry mouth or throat.
- Skin rash, itching.
- Increased levels of liver enzymes in the blood, which can be observed with a blood test.
- Fatigue.
- Benign stomach polyps.
Uncommon(may affect up to 1 in 100 people):
- Depression.
- Pain in muscles or joints.
- Fracture of the hip, wrist, or spine.
- Fluid retention or swelling.
- Changes in blood cell count.
Rare(may affect up to 1 in 1,000 people):
- Fever.
- Pain or difficulty swallowing with white spots on the back of the throat. These symptoms could be indicative of a fungal infection in the esophagus.
- Restlessness, drowsiness.
- Confusion, seeing, feeling, or hearing things that do not exist (hallucinations), sleep problems (insomnia).
- Visual disturbances.
- Sensation of rotational movement of the body when standing (vertigo).
- Alteration of taste, loss of appetite, tongue inflammation (glossitis).
- Skin reactions such as burning or itching sensation under the skin, bruising, redness, and excessive sweating.
- Skin sensitivity to light.
- Alopecia (hair loss).
- Sensation of tingling (paresthesia), tremors.
- Anemia (pallor).
- Swelling of the breasts in males, impotence.
Very Rare(may affect up to 1 in 10,000 people):
- Mouth inflammation (stomatitis).
- Decrease in sodium levels in the blood, symptoms include nausea and vomiting, headache, drowsiness, and fatigue, confusion, muscle weakness or spasms, irritability, convulsions, coma. Your doctor may decide to perform periodic blood tests to check your sodium levels.
- Increased levels of cholesterol or lipids in the blood (observed through blood tests).
Frequency Not Known(cannot be estimated from available data):
- Visual hallucinations.
- If you have been taking lansoprazole for more than three months, it is possible that your magnesium levels in the blood may decrease. Low magnesium levels can cause fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness, or increased heart rate. If you experience any of these symptoms, inform your doctor immediately. Low magnesium levels can also cause a decrease in potassium or calcium levels in the blood. Your doctor may decide to perform periodic blood tests to check your magnesium levels.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Lansoprazol Flas Viatris
Keep this medicine out of sight and reach of children.
Store in the original packaging to protect it from moisture.
Bottles: Once opened, use within 100 days. Keep the bottle perfectly closed to protect its contents from moisture.
Do not use this medicine after the expiration date that appears on the blister pack, carton, and bottle after CAD or EXP. The expiration date is the last day of the month indicated.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Lansoprazol Flas Viatris
The active ingredient is lansoprazole.
The other ingredients are: sugar spheres, light magnesium carbonate (E-504), crospovidone (E-1202), hydroxypropylcellulose (E-463), methacrylic acid and ethyl acrylate copolymer (1:1), triethyl citrate (E-1505), sodium hydroxide (E-524), talc (E-553b), polysorbate (E-433), macrogol, red iron oxide (E-172), yellow iron oxide (E-172), mannitol (E-421), microcrystalline cellulose (E-460), sodium carboxymethyl starch type A (derived from potato starch), aspartame (E-951), sodium lauryl sulfate, sodium bicarbonate (E-500), citric acid monohydrate (E-330), strawberry flavor [flavoring, corn maltodextrin, and propylene glycol (E-152)], and magnesium stearate. (See section 2 "Lansoprazol Flas Viatris contains sucrose, aspartame, and sodium").
Appearance of the Product and Package Contents
The medicine is presented in the form of orodispersible tablets (solid oral formulation) that dissolve to release gastro-resistant microgranules.
Lansoprazol Flas Viatris 15 mg are round, white to yellowish tablets with orange to dark brown speckles, flat, with a beveled edge, marked with "LP1" on one side and "M" on the other.
Lansoprazol Flas Viatris 15 mg orodispersible tablets are available in:
- Peelable blisters of 7, 14, 28, 30, 56, 90, or 98 tablets.
- Perforated unit-dose peelable blisters of 28 tablets.
- Press-through blisters of 7, 14, 28, 30, 56, 90, or 98 tablets.
- Perforated unit-dose press-through blisters of 28 tablets.
- Plastic bottles with hydrophilic cotton and screw cap containing 30, 100, or 500 tablets.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Viatris Limited
Damastown Industrial Park
Mulhuddart, Dublin 15
Dublin
Ireland
Manufacturer
Generics [UK] Ltd
Station Close, Potters Bar, Hertfordshire, EN6 1TL
United Kingdom
or
McDermott Laboratories Limited trading as Gerard Laboratories
35/36 Baldoyle Industrial Estate, Grange Road, Dublin 13
Ireland
or
Mylan Hungary Kft.
H-2900, Komárom Mylan utca 1
Hungary
or
Mylan UK Healthcare Limited
Building 20, Station Close, Potters Bar, EN6 1TL
United Kingdom
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Viatris Pharmaceuticals, S.L.U.
C/ General Aranaz, 86
28027 - Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area and the United Kingdom (Northern Ireland) with the following names:
Spain Lansoprazol Flas Viatris 15 mg orodispersible tablets EFG
France Lansoprazole Viatris 15 mg orodispersible tablet
Italy Lansoprazolo Mylan Generics Italia
Malta Lansoprazole 15 mg orodispersible tablets
Portugal Lansoprazole Mylan
United Kingdom Lansoprazole 15 mg Orodispersible tablets
Sweden Lansoprazol Viatris 15 mg muns?nderfallande tablett
Date of the Last Revision of this Leaflet:July 2023
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.96 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LANSOPRAZOL FLAS VIATRIS 15 mg ORALLY DISINTEGRATING TABLETSDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 15 mgActive substance: lansoprazoleManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 30 mgActive substance: lansoprazoleManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: CAPSULE, 15 mg lansoprazoleActive substance: lansoprazoleManufacturer: Merck S.L.Prescription required
Online doctors for LANSOPRAZOL FLAS VIATRIS 15 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about LANSOPRAZOL FLAS VIATRIS 15 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















