KAYFANDA 1200 micrograms hard capsules

How to use KAYFANDA 1200 micrograms hard capsules
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
KAYFANDA 200micrograms hard capsules
KAYFANDA 400micrograms hard capsules
KAYFANDA 600micrograms hard capsules
KAYFANDA 1200micrograms hard capsules
odevixibat
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4).
Contents of the pack
- What is KAYFANDA and what is it used for
- What you need to know before you take KAYFANDA
- How to take KAYFANDA
- Possible side effects
- Storing KAYFANDA
- Contents of the pack and other information
1. What is KAYFANDA and what is it used for
What is KAYFANDA
KAYFANDA contains the active substance odevixibat. This medicine helps with the elimination of bile acids, which are found in a digestive fluid called bile, a fluid that is produced in the liver and helps to break down fats in the intestine. After helping with digestion, bile acids are reabsorbed from the intestine and return to the liver.
What KAYFANDA is used for
KAYFANDA is used to treat itching caused by the buildup of bile in patients from 6 months of age who have Alagille syndrome (ALGS).
ALGS is a rare genetic disease that can affect many parts of the body, such as the liver, heart, eyes, face, skeleton, blood vessels, and kidneys. Patients with this syndrome have a reduced bile flow, which causes a buildup of bile acids in the blood and liver (cholestasis) that worsens over time and is usually accompanied by intense itching.
How KAYFANDA works (odevixibat)
The active substance in KAYFANDA, odevixibat, reduces the absorption of bile acids from the intestine. This allows them to be eliminated from the body through the feces, preventing their buildup in the liver. In this way, odevixibat reduces the amount of bile acids present in the blood.
2. What you need to know before you take KAYFANDA
Do not take KAYFANDA
- if you are allergic to odevixibat or any of the other ingredients of this medicine (listed in section 6)
Warnings and precautions
Talk to your doctor or pharmacist before starting to take KAYFANDA if you have:
- severe liver dysfunction;
- a reduction in stomach or intestinal function or a reduction in bile acid flow between the liver, gallbladder, and small intestine due to medications, surgical interventions, or diseases other than ALGS. All of this can decrease the effect of odevixibat.
Talk to your doctor if you have diarrhea while taking KAYFANDA. If you have diarrhea, drink plenty of fluids to avoid dehydration.
During treatment with KAYFANDA, an increase in liver enzyme levels may be observed in liver function tests. Your doctor will monitor your liver function before and during treatment with KAYFANDA. Your doctor may recommend more frequent checks if you have elevated test results. Before and during treatment, your doctor may also check your blood levels of vitamins A, D, and E and your INR (international normalized ratio, which measures your risk of bleeding).
Children
KAYFANDA is not recommended for use in children under 6 months of age, as it is not known if the medicine is safe and effective in this age group.
Other medicines and KAYFANDA
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Treatment with odevixibat may affect the absorption of fat-soluble vitamins, such as vitamins A, D, and E.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
KAYFANDA is not recommended during pregnancy or in women of childbearing age who are not using contraceptive methods.
It is not known if odevixibat can pass into breast milk and affect the baby. Your doctor will help you decide whether to stop breastfeeding or avoid treatment with odevixibat, considering the benefit of breastfeeding for the baby and the benefit of odevixibat for the mother.
Driving and using machines
KAYFANDA has no influence or negligible influence on the ability to drive and use machines.
3. How to take KAYFANDA
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Treatment should be initiated and supervised by a doctor with experience in the treatment of progressive liver disease with reduced bile flow.
How much KAYFANDA to take
- The dose of odevixibat depends on your weight. Your doctor will calculate the appropriate dose and concentration of the capsules you should take.
- The recommended dose is 120 micrograms of odevixibat per kilogram of body weight once a day (up to a maximum of 7,200 micrograms once a day). Your doctor may recommend reducing the dose to 40 micrograms of odevixibat per kilogram of body weight once a day if you have diarrhea that lasts ≥ 3 days, if it is considered intense, or if it requires IV hydration.
If the medicine does not improve your condition after 6 months of continuous daily treatment, your doctor will recommend an alternative treatment.
How to use KAYFANDA
Take the capsules once a day in the morning, with or without food.
All capsules can be swallowed whole with a glass of water or opened and sprinkled over some food or in a liquid suitable for the age (e.g., breast milk, formula, or water).
The larger capsules, 200 and 600 micrograms, are designed to be opened and sprinkled over some food or in a liquid suitable for the age, but can be swallowed whole.
The smaller capsules, 400 and 1,200 micrograms, are designed to be swallowed whole, but can be opened and sprinkled over some food or in a liquid suitable for the age.
You will find detailed instructions on how to open the capsules and sprinkle the contents over some food or in a liquid at the end of this leaflet.
If you take more KAYFANDA than you should
Tell your doctor if you think you have taken too much KAYFANDA.
Possible symptoms of an overdose are diarrhea and stomach and intestine problems.
If you forget to take KAYFANDA
If you forget to take KAYFANDA, take the missed dose as soon as possible, but do not take more than one dose a day.
If you stop taking KAYFANDA
Do not stop taking KAYFANDA without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects may occur with the following frequencies:
very common(may affect more than 1 in 10 people)
- diarrhea
common(may affect up to 1 in 10 people)
- abdominal pain (in the belly)
- vomiting
- increased liver enzyme levels, measured in blood tests
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4). You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing KAYFANDA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of the month shown.
Store in the original package to protect from light. Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What KAYFANDA contains
- The active substance is odevixibat.
Each hard capsule of KAYFANDA 200 micrograms contains 200 micrograms of odevixibat (as sesquihydrate).
Each hard capsule of KAYFANDA 400 micrograms contains 400 micrograms of odevixibat (as sesquihydrate).
Each hard capsule of KAYFANDA 600 micrograms contains 600 micrograms of odevixibat (as sesquihydrate).
Each hard capsule of KAYFANDA 1,200 micrograms contains 1,200 micrograms of odevixibat (as sesquihydrate).
- The other ingredients are:
Capsule content
Microcrystalline cellulose, hypromellose
Capsule shell
KAYFANDA 200micrograms and 600micrograms hard capsules
Hypromellose, titanium dioxide (E171), yellow iron oxide (E172)
KAYFANDA 400micrograms and 1200micrograms hard capsules
Hypromellose, titanium dioxide (E171), yellow iron oxide (E172), red iron oxide (E172)
Printing ink
Lacquer, propylene glycol, black iron oxide (E172)
Appearance and packaging
KAYFANDA 200 micrograms hard capsules:
Capsule size 0 (21.7 mm × 7.64 mm) with opaque ivory cap and opaque white body; with inscription «A200» in black ink.
KAYFANDA 400 micrograms hard capsules:
Capsule size 3 (15.9 mm × 5.82 mm) with opaque orange cap and opaque white body; with inscription «A400» in black ink.
KAYFANDA 600 micrograms hard capsules:
Capsule size 0 (21.7 mm × 7.64 mm) with opaque ivory cap and body; with inscription «A600» in black ink.
KAYFANDA 1,200 micrograms hard capsules:
Capsule size 3 (15.9 mm × 5.82 mm) with opaque orange cap and body; with inscription «A1200» in black ink.
KAYFANDA hard capsules are packaged in a plastic bottle (high-density polyethylene (HDPE) bottle) with a polypropylene closure, with a security seal and child-resistant closure. Pack size: 30 hard capsules.
Marketing authorisation holder
Ipsen Pharma
65 quai Georges Gorse
92100 Boulogne-Billancourt
France
Manufacturer
Almac Pharma Services Limited
Seagoe Industrial Estate
Portadown, Craigavon
County Armagh
BT63 5UA
United Kingdom (Northern Ireland)
Date of last revision of this leaflet
This medicine has been authorised under exceptional circumstances. This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicine.
The European Medicines Agency will review any new information that may become available every year and this leaflet will be updated as necessary.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions
Instructions for opening the capsules and sprinkling the contents over some food
Step 1. Put a small amount of soft food in a bowl (2 tablespoons/30 ml of yogurt, apple sauce, banana puree, or zanahoria, chocolate pudding, rice pudding, or oatmeal). The food should be at room temperature or cooler.
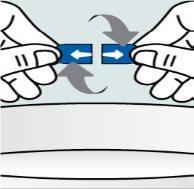
| Step 2:
ends and twist in opposite directions. |
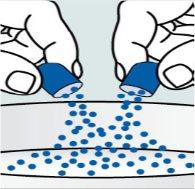
| Step 3:
that contains the soft food.
come out.
needed for the dose. |
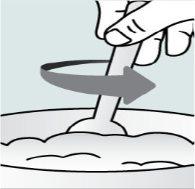
| Step 4:
food. |
|
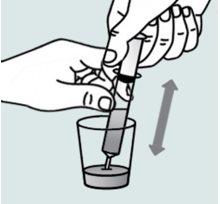
Instructions for opening the capsules and sprinkling the contents in a liquid suitable for the age
Contact your pharmacy if you do not have an appropriate oral syringe to administer the medicine at home.
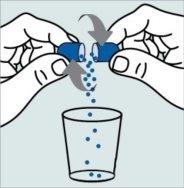
| Step 1:
and twist in opposite directions.
cup or glass. The granules will not pass through the opening of baby bottles or cups.
come out. Repeat this step if more than one capsule is needed for the dose. |
(e.g., breast milk, formula, or water).
minutes to allow them to become completely wet (the granules will not dissolve in liquids). | |
| Step 2:
into the mixture glass.
granule mixture into the syringe.
liquid and granule mixture to the mixture glass. Repeat this process two or three times to ensure complete mixing of the granules in the liquid. |
Step 3:
the plunger up to the end of the syringe. | |
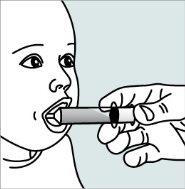
| Step 4:
child's mouth between the tongue and the side of the mouth, and then gently push the plunger down to administer a stream of the liquid and granule mixture between the tongue and the side of the child's mouth. Do not administer a stream of the liquid and granule mixture into the back of the child's throat, as this could cause gagging or choking. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KAYFANDA 1200 micrograms hard capsulesDosage form: CAPSULE, 1200 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription requiredDosage form: CAPSULE, 200 µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription requiredDosage form: CAPSULE, 200 - REVIEW µgActive substance: odevixibatManufacturer: Ipsen PharmaPrescription required
Online doctors for KAYFANDA 1200 micrograms hard capsules
Discuss questions about KAYFANDA 1200 micrograms hard capsules, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions