
HIBOR 3,500 IU Anti-Xa / 0.2 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use HIBOR 3,500 IU Anti-Xa / 0.2 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
HIBOR 3,500 IU anti-Xa/0.2 ml
Injectable solution in pre-filled syringes
Bemiparin sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is HIBOR 3,500 IU and what is it used for
- What you need to know before you use HIBOR 3,500 IU
- How to use HIBOR 3,500 IU
- Possible side effects
- Storage of HIBOR 3,500 IU
- Contents of the pack and further information
1. What is HIBOR 3,500 IU and what is it used for
The active substance of HIBOR is bemiparin sodium, which belongs to a group of medicines called anticoagulants. These medicines help prevent blood from clotting in the veins.
HIBOR 3,500 IU is used to: prevent blood clots (for example, in the veins of the legs and/or lungs) that may appear in patients undergoing orthopedic surgery, in patients not undergoing surgical intervention but who have a high risk of suffering clots, and in patients who have already suffered blood clots and have transient risk factors.
It is also used to prevent clot formation in the extracorporeal circulation circuit during hemodialysis.
2. What you need to know before you start using HIBOR 3,500 IU
Do not use HIBOR 3,500 IU
- If you are allergic to bemiparin sodium, heparin, or a similar product (such as enoxaparin, dalteparin, or nadroparin) or to any of the other components of this medicine (listed in section 6).
- If you have had an allergic reaction after being treated with a medicine that contains heparin.
- If you are allergic to any substance derived from pigs.
- If you have Heparin-Induced Thrombocytopenia (HIT), a disease that causes a significant decrease in your platelet count (or, as a result of HIT, you suffer from another disease called Disseminated Intravascular Coagulation (DIC), in which your platelets would clump if you use HIBOR).
- If you have a disease called endocarditis (inflammation of the heart walls and heart valves).
- If you have any type of disorder that causes you to bleed excessively.
- If you have a severe liver or pancreas disorder.
- If you suffer from any type of damage or injury to your internal organs that could imply a high risk of internal bleeding (for example, active stomach ulcers, cerebral aneurysms [inflammation of the walls of the brain arteries] or brain tumors).
- If you have had a cerebral hemorrhage.
- If you have had or have a lesion or are going to be operated on in the brain, spinal cord, eyes, and/or ears in the last 2 months.
- If you are using HIBOR, you will not be able to receive epidural or spinal anesthesia (an anesthetic injected into the spinal cord) because it could be dangerous. Therefore, make sure your doctor knows that you are using HIBOR before any operation.
Warnings and precautionsConsult your doctor before starting to use HIBOR 3,500 IU
- If you are sick with liver disease.
- If you are sick with kidney disease. Your doctor may consider special monitoring. In case your disease is severe, your doctor may consider it necessary to adjust the dose.
- If your blood pressure is high and/or difficult to control.
- If you have ever had a stomach ulcer that is no longer active.
- If you have thrombocytopenia, a disease in which there are fewer platelets than normal in the blood, which causes bruising and easy bleeding.
- If you have kidney or bladder stones.
- If you have any type of disease that causes you to bleed easily.
- If you have eye problems due to blood vessel problems.
- If you have diabetes.
- If your test results have shown that you have high potassium levels in your blood.
- Make sure your doctor knows that you are using HIBOR if you are going to have a lumbar puncture (a puncture in the lower back for analysis).
Using HIBOR 3,500 IU with other medicines
Tell your doctor if you are using, have recently used, or may need to use any other medicine.
- Any medicine that is injected into the muscle, because these injections should be avoided while you are being treated with HIBOR.
- Other anticoagulants such as warfarin and/or acenocoumarol (vitamin K antagonists) to treat and/or prevent blood clots.
- Non-steroidal anti-inflammatory drugs, such as ibuprofen, for example, for arthritis.
- Corticosteroids such as prednisolone, to treat inflammatory diseases, such as arthritis.
- Platelet inhibitors, such as aspirin, ticlopidine, or clopidogrel, to prevent blood clots.
- Medicines that may increase potassium levels in the blood, such as some diuretics and antihypertensives (used to reduce blood pressure).
- Medicines to increase blood volume, such as dextran.
- An injectable medicine for heart problems called nitroglycerin.
Special tests you may need
- Some patients may need to have their platelet levels controlled. Your doctor will decide if it is necessary and when (for example, before starting treatment, on the first day of treatment, then every 3 or 4 days until the end of treatment).
- If you have certain diseases (diabetes, kidney disease) or if you are taking medicines to prevent potassium loss, your doctor may monitor your potassium levels in your blood.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
HIBOR does not affect your ability to drive and use machines.
3. How to use HIBOR 3,500 IU
Follow exactly the administration instructions of this medicine indicated by your doctor, pharmacist, or nurse. In case of doubt, ask your doctor, pharmacist, or nurse.
HIBOR is injected under the skin, usually in a skin fold on one side of the waist (abdomen) or in the upper part of the hip. Normally, your doctor or nurse will give you the injection in the hospital. You may need to continue receiving HIBOR when you go home.
This medicine should never be injected into a muscle or mixed with any other injection. It is usually administered once a day.
Your doctor will tell you how long you should be given this medicine.
If your doctor has told you that you can inject this medicine yourself, follow your doctor's instructions carefully (see section "How do I inject HIBOR?").
The recommended dose is:
Adults (18-64 years)
Undergoing orthopedic surgery:
- You will receive a dose of the product (the contents of one syringe = 3,500 IU) before or after your operation.
- In the following days, you will receive a daily dose of the product (the contents of one syringe = 3,500 IU).
At high risk of suffering clots:
- You will receive a daily dose of the product (the contents of one syringe = 3,500 IU) for the period established by your doctor.
Who have suffered blood clots previously:
- You will receive a daily dose of the product (the contents of one syringe = 3,500 IU) for a maximum period of 3 months.
UI: the potency of this medicine is described in international units of anti-Xa activity.
Elderly(from 65 years)
They usually receive the same dose as other adult patients. If you have liver or kidney problems, please inform your doctor, it is possible that they will decide to monitor you very closely. If your kidney disease is mild or moderate, no dose adjustment is considered necessary.
Patients with renal insufficiency
In case your kidney disease is severe, your doctor may consider it necessary to adjust the dose.
If your kidney disease is mild or moderate, no dose adjustment is necessary.
Patients with hepatic insufficiency
There is not enough data for your doctor to recommend a dose adjustment.
Use in children and adolescents
HIBOR is not recommended in children.
How do I inject HIBOR?
HIBOR should never be injected into a muscle because it could cause bleeding inside the muscle. Before giving yourself your first injection, you should receive instructions on the correct use of this medicine and on the correct technique for self-injection. These instructions should be given by a doctor or another qualified healthcare professional.
You should follow these steps:
- Wash your hands thoroughly and sit or lie down in a comfortable position.
- Choose a area of the waist that is at least 5 centimeters from the navel and from any scar or bruise, and clean the skin of that area thoroughly.
- Use a different site for the injection each day, for example, first on the left side and the next time on the right.
- Remove the cap that covers the needle of the HIBOR syringe.
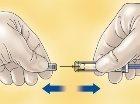
- To keep the needle sterile, make sure it does not touch anything.
- The pre-filled syringe is ready to use.
- Before the injection, do not push the plunger to eliminate air bubbles, because you may lose medicine.
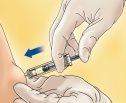
- Hold the syringe with one hand and with the other, using your index and thumb fingers, hold a pinch of the cleaned skin area to form a fold.
- Insert the entire needle into the skin fold, keeping the syringe as upright as possible over the body surface, at a 90-degree angle.
- Push the plunger, making sure to keep the skin fold in the same position until the plunger is all the way down.
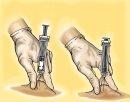
- Remove the syringe from the injection site, keeping your finger on the plunger and the syringe upright. Release the skin fold.
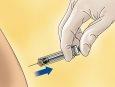
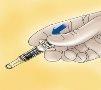
- For syringes with a safety device: Direct the needle away from you and anyone present, activate the safety system by pressing firmly on the plunger. The protective cover will automatically cover the needle and you will hear a click that will confirm the activation of the protector.
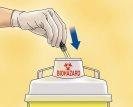
- Immediately discard the syringe by throwing it into the nearest sharps container (needle first), close the container tightly with the lid, and keep it out of the reach of children.
Warnings:
- The safety system can only be activated once the syringe has been emptied.
- Activation of the safety system should only be performed after the needle has been removed from the patient's skin.
- Do not reuse the needle protection after injection.
- Activation of the safety system may splash a minimal amount of liquid. For your maximum safety, activate the safety system by directing it downwards and away from you and anyone present.
- Do not rub the skin where the injection was given. This will help prevent bruising.
If you think the effect of HIBOR 3,500 IU is too strong (for example, because you experience unexpected bleeding) or too weak (for example, because the dose does not seem to work), tell your doctor or pharmacist.
If you use more HIBOR 3,500 IU than you should
This can cause bleeding. In this case, consult your doctor or go to the emergency department of the nearest hospital with this leaflet.
In case of overdose or accidental administration, call the Toxicology Information Service. Phone 91 562 04 20, indicating the medicine and the amount administered.
If you forget to use HIBOR 3,500 IU
Do not use a double dose to make up for forgotten doses. If this happens, you should consult your doctor as soon as possible to find out what to do.
If you stop using HIBOR
Always consult your doctor before stopping this medicine.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using HIBOR and consult your doctor or nurse (or go immediately to the emergency department of the nearest hospital) if you suffer from any of the following side effects:
Common(may affect up to 1 in 10 people):
- Unusual or unexpected bleeding, for example, blood in the urine or stool, which can cause hemorrhagic anemia.
Rare(may affect up to 1 in 1,000 people):
- Severe decrease in the number of platelets (Type II Thrombocytopenia), which can lead to the appearance of bruising, bleeding gums, nose, and mouth, and rashes.
- Skin damage (necrosis) at the injection sites.
- If you have undergone a lumbar puncture or have received epidural or spinal anesthesia, HIBOR can cause bleeding in the spinal cord and the formation of hematomas. This could cause loss of strength or sensation in the legs and lower part of your body and/or incontinence of feces and urine. These hematomas can cause different degrees of disability, including prolonged or permanent paralysis. If this happens, stop using HIBOR 3,500 IU and inform your doctor or nurse immediately.
- Severe allergic reactions (fever, shivering, difficulty breathing, swelling of the vocal cords, dizziness, sweating, hives, rash, itching, low blood pressure, hot flashes, redness, fainting, bronchial constriction, laryngeal edema).
Other side effects:
Very common (affects more than 1 in 10 people):
- Bruises, skin spots, itching, and some pain in the areas where you injected the medicine.
Common (may affect up to 1 in 100 people):
- A mild and transient increase in certain liver enzymes (transaminases) in the blood, which may appear in blood tests.
Uncommon (may affect up to 1 in 1,000 people):
- Mild allergic reactions on the skin (rash, skin rash, hives, itching, urticaria).
- Mild and transient decrease in the number of platelets (Type I Thrombocytopenia), which may appear in blood tests.
Frequency not known (cannot be estimated from the available data):
- Hyperkalemia (increased potassium levels in the blood).
- Bone fragility (osteoporosis) associated with prolonged treatment with heparin.
Reporting of side effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of HIBOR 3,500 IU
Keep this medicine out of the sight and reach of children.
Do not store above 30°C. Do not freeze.
Do not use this medicine if you notice:
- The protective packaging is open.
- The protective packaging is damaged.
- The medicine contained in the syringe is cloudy.
- Small particles in the medicine.
Once the blister pack containing the syringe is opened, the medicine should be used immediately.
Expiry date
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date is the last day of the month indicated.
Disposal
This medicine is presented in single-use syringes.
Dispose of used syringes in a sharps container.
Do not store them after use.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and medicines you no longer need in the SIGRE Point of the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Additional Information
Composition of HIBOR 3,500 UI
The active ingredient is: Sodium Bemiparin 3,500 UI.
The other components are: Water for injections.
Appearance of the product and packaging content
The medication contained in the syringes is a clear, colorless or slightly yellowish solution, without visible particles.
HIBOR 3,500 UI is available in boxes of 2, 10, 30, and 50 pre-filled syringes. Each syringe contains 0.2 ml of solution. Each 0.2 ml syringe provides a dose of sodium bemiparin of 3,500 UI.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
LABORATORIOS FARMACÉUTICOS ROVI, S.A.
C/ Julián Camarillo, 35
28037 MADRID
Manufacturer
ROVI Pharma Industrial Services, S.A.
C/ Julián Camarillo, 35
28037 MADRID
LABORATORIOS FARMACÉUTICOS ROVI, S.A.
C/ Julián Camarillo, 35
28037 MADRID
Date of the last revision of this prospectus: 05/2023
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price54.58 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HIBOR 3,500 IU Anti-Xa / 0.2 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 10000/IUActive substance: bemiparinManufacturer: Laboratorios Farmaceuticos Rovi S.A.Prescription requiredDosage form: INJECTABLE, 12,500 IUActive substance: bemiparinManufacturer: Laboratorios Farmaceuticos Rovi S.A.Prescription requiredDosage form: INJECTABLE, 2500 IUActive substance: bemiparinManufacturer: Laboratorios Farmaceuticos Rovi S.A.Prescription required
Online doctors for HIBOR 3,500 IU Anti-Xa / 0.2 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about HIBOR 3,500 IU Anti-Xa / 0.2 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















