FIRMAGON 120 mg POWDER AND SOLVENT FOR INJECTION


How to use FIRMAGON 120 mg POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
FIRMAGON 120mgpowder and solvent for solution for injection
degarelix
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is FIRMAGON and what is it used for
- What you need to know before you use FIRMAGON
- How to use FIRMAGON
- Possible side effects
- Storage of FIRMAGON
- Contents of the pack and further information
1. What is FIRMAGON and what is it used for
FIRMAGON contains degarelix.
Degarelix is a synthetic hormonal blocker used for the treatment of cancer and for the treatment of high-risk prostate cancer before radiotherapy and in combination with radiotherapy in adult male patients. Degarelix mimics the effects of a natural hormone (gonadotropin-releasing hormone, GnRH) by directly blocking its effects. For this reason, degarelix rapidly reduces the levels of the male hormone testosterone, which is responsible for stimulating prostate cancer.
2. What you need to know before you use FIRMAGON
Do not use FIRMAGON
- If you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor if you have:
- Any vascular condition or heart rhythm problems (arrhythmias) or if you are being treated with medicines to correct this condition. The risk of heart rhythm problems may be increased with the use of FIRMAGON.
- Diabetes mellitus. Worsening or onset of diabetes may occur. If you have diabetes, you may need to check your blood sugar levels more often.
- Liver disease. You may need to have your liver function monitored.
- Kidney disease. The use of FIRMAGON has not been investigated in patients with severe kidney disease.
- Osteoporosis or any condition that affects bone density. Reduced testosterone levels can cause a reduction in bone calcium (thinning of the bones).
- Severe hypersensitivity. The use of FIRMAGON has not been investigated in patients with severe hypersensitivity reactions.
Children and adolescents
Do not give this medicine to children or adolescents.
Using FIRMAGON with other medicines
FIRMAGON may interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone, and sotalol) or with medicines that affect heart rhythm (e.g. methadone (used for pain relief and as part of drug detoxification), moxifloxacin (an antibiotic), antipsychotics).
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Driving and using machines
Fatigue and dizziness are common side effects that may affect your ability to drive and use machines. These side effects may be due to treatment or may be derived from the underlying disease.
3. How to use FIRMAGON
Generally, the injection of this medicine will be performed by a nurse or doctor.
The recommended starting dose is two consecutive injections of 120 mg. After that, you will be injected with a monthly dose of 80 mg. The liquid that is injected forms a gel from which degarelix is released over a month.
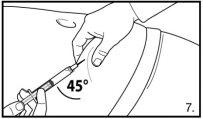
FIRMAGON SHOULD ONLY be injected under the skin (subcutaneous injection). FIRMAGON SHOULD NOT be administered into a blood vessel (intravenous injection). Particular care should be taken to avoid accidental injection into a vein. It is usual to vary the injection site at different points on the abdominal wall.
If you miss a dose of FIRMAGON
If you think you have missed your monthly dose of FIRMAGON, ask your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, FIRMAGON can cause side effects, although not everybody gets them.
A very severe allergic reaction to this medicine is rare. Contact your doctor immediately if you develop a severe skin rash, itching, or difficulty breathing. This could be a symptom of a severe allergic reaction.
Very common (may affect more than 1 in 10 people)
Hot flushes, injection site reactions, and redness. Injection site reactions occur more frequently with the starting dose and are less frequent when administering the maintenance dose.
Common (may affect up to 1 in 10 people)
- Swelling, nodule, and hardness at the injection site
- Chills, fever, or flu-like symptoms after injection
- Difficulty sleeping, fatigue, dizziness, headache
- Weight gain, nausea, diarrhea, increased liver enzymes
- Excessive sweating (including night sweats), skin rash
- Anemia
- Musculoskeletal pain and discomfort
- Reduced testicle size, breast tenderness, impotence
Uncommon (may affect up to 1 in 100 people)
- Loss of sexual desire, testicular pain, pelvic pain, interruption of ejaculation, genital irritation, chest pain
- Depression, mental impairment
- Skin discoloration, hair loss, skin nodules, numbness
- Allergic reactions, hives, itching
- Decreased appetite, constipation, vomiting, dry mouth, abdominal pain and discomfort, increased blood sugar/diabetes mellitus, increased cholesterol, changes in blood calcium levels, weight loss
- High blood pressure, changes in heart rhythm, changes in electrocardiogram (QT prolongation), abnormal heart pumping sensation, shortness of breath, peripheral edema
- Muscle weakness, muscle spasms, joint swelling/stiffness, osteoporosis/osteopenia, joint pain
- Frequent urination, urgent urination (sudden need to urinate), difficulty or pain when urinating, need to urinate at night, kidney function impairment, incontinence
- Blurred vision
- Injection site reaction including decreased blood pressure and heart rate (vasovagal reaction)
- Discomfort
Rare (may affect up to 1 in 1,000 people)
- Febrile neutropenia (very low white blood cell count in the blood in combination with fever), heart attack, heart failure
- Unexplained muscle pain or cramps, sensitivity, or weakness. Muscle problems can be severe, including muscle breakdown that can damage the kidneys.
Very rare (may affect up to 1 in 10,000 people)
- Injection site infection, abscess, and necrosis
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of FIRMAGON
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vials, syringes, and carton. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
After reconstitution
This medicine is stable for 2 hours at 25°C.
Due to the risk of microbiological contamination, the medicine should be used immediately. If not used immediately, the use of this medicine will be the responsibility of the user.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of FIRMAGON
- The active substance is degarelix. Each vial contains 120 mg of degarelix (as acetate). After reconstitution, 1 ml of reconstituted solution contains 40 mg of degarelix.
- The other ingredient of the powder is mannitol (E 421).
- The solvent is water for injections.
Appearance and pack contents of FIRMAGON
FIRMAGON is a powder and solvent for solution for injection. The powder is white to off-white. The solvent is a clear and colorless solution.
Pack size of 2 trays containing:
2 vials of powder containing 120 mg of degarelix and 2 pre-filled syringes containing 3 ml of solvent.
2 plunger stoppers, 2 vial adapters, and 2 needles for injection.
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
Ferring Pharmaceuticals A/S
Amager Strandvej 405
2770 Kastrup, Denmark
Tel. +45 8833 8834
Manufacturer:
Ferring GmbH
Wittland 11
D-24109 Kiel, Germany
You can request more information about this medicine from the local representative of the marketing authorisation holder:
Belgium Ferring N.V. Tel: +32 53 72 92 00 | Lithuania CentralPharma Communication UAB Tel: +370 5 243 0444 |
Bulgaria Farmont Ltd. Tel: +359 2 807 5022 | Luxembourg Ferring N.V. Belgium Tel: +32 53 72 92 00 |
Czech Republic Ferring Pharmaceuticals CZ s.r.o. Tel: +420 234 701 333 | Hungary Ferring Magyarország Gyógyszerkereskedelmi Kft. Tel: +36 1 236 3800 |
Denmark Ferring Lægemidler A/S Tel: +45 88 16 88 17 | Malta E.J. Busuttil Ltd. Tel: +356 21447184 |
Germany Ferring Arzneimittel GmbH Tel: +49 431 5852 0 | Netherlands Ferring B.V. Tel: +31 235680300 |
Estonia CentralPharma Communication OÜ Tel: +372 601 5540 | Norway Ferring Legemidler AS Tel: +47 22 02 08 80 |
Greece Ferring Ελλάς ΜΕΠΕ Tel: +30 210 68 43 449 | Austria Ferring Arzneimittel Ges.m.b.H. Tel: +43 1 60 8080 |
Spain Ferring, S.A.U. Tel: +34 91 387 70 00 | Poland Ferring Pharmaceuticals Poland Sp. z o.o. Tel: +48 22 246 06 80 |
France Ferring S.A.S. Tel: +33 1 49 08 67 60 | Portugal Ferring Portuguesa – Produtos Farmacêuticos, Sociedade Unipessoal, Lda. Tel: +351 21 940 5190 |
Croatia Clinres farmacija d.o.o. Tel: +385 1 2396 900 | Romania Ferring Pharmaceuticals Romania SRL Tel: +40 356 113 270 |
Ireland Ferring Ireland Ltd. Tel: + 353 1 4637355 | Slovenia SALUS, Veletrgovina, d.o.o. Tel: +386 1 5899 179 |
Iceland Vistor hf. Tel: +354 535 70 00 | Slovakia Ferring Slovakia s.r.o. Tel: +421 2 54 416 010 |
Italy Ferring S.p.A. Tel: +39 02 640 00 11 | Finland Ferring Lääkkeet Oy Tel: +358 207 401 440 |
Cyprus
Tel: +357 22583333 | Sweden Ferring Läkemedel AB Tel: +46 40 691 69 00 |
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency web site http://www.ema.europa.eu/.
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Instructions for correct use
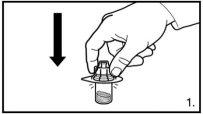
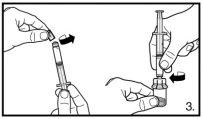
NOTE:
- THE VIALS SHOULD NOT BE SHAKEN
The pack contains two vials of powder and two pre-filled syringes with solvent that need to be prepared for subcutaneous injection. Therefore, the procedure described should be repeated a second time.
|
|
| |
|
|
|
Small circular air bubbles may be acceptable. The reconstitution process usually takes a few minutes, but in some cases, it may take up to 15 minutes. |
|
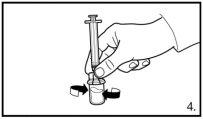
Always make sure to withdraw the exact volumeand adjust if air bubbles form |
| |
|
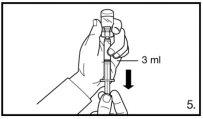
Inject 3 ml of FIRMAGON 120mgslowly, immediately after reconstitution* |
Do not inject directly into any vein. Gently pull back the plunger to check if blood has been aspirated. If blood enters the syringe, the product cannot be used. In this case, remove and discard the syringe and needle (reconstitute a new dose for the patient). | |
|
- Chemical and physical stability of the reconstituted product has been demonstrated for 2 hours at 25°C. From a microbiological point of view, unless the method of reconstitution implies a risk of contamination, the product should be used immediately. If not used immediately, the conditions and duration of use will be the responsibility of the user.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FIRMAGON 120 mg POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, 80 mgActive substance: degarelixManufacturer: Ferring Pharmaceuticals A/SPrescription requiredDosage form: TABLET, 250 mgActive substance: abirateroneManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 500 mgActive substance: abirateroneManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for FIRMAGON 120 mg POWDER AND SOLVENT FOR INJECTION
Discuss questions about FIRMAGON 120 mg POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions