
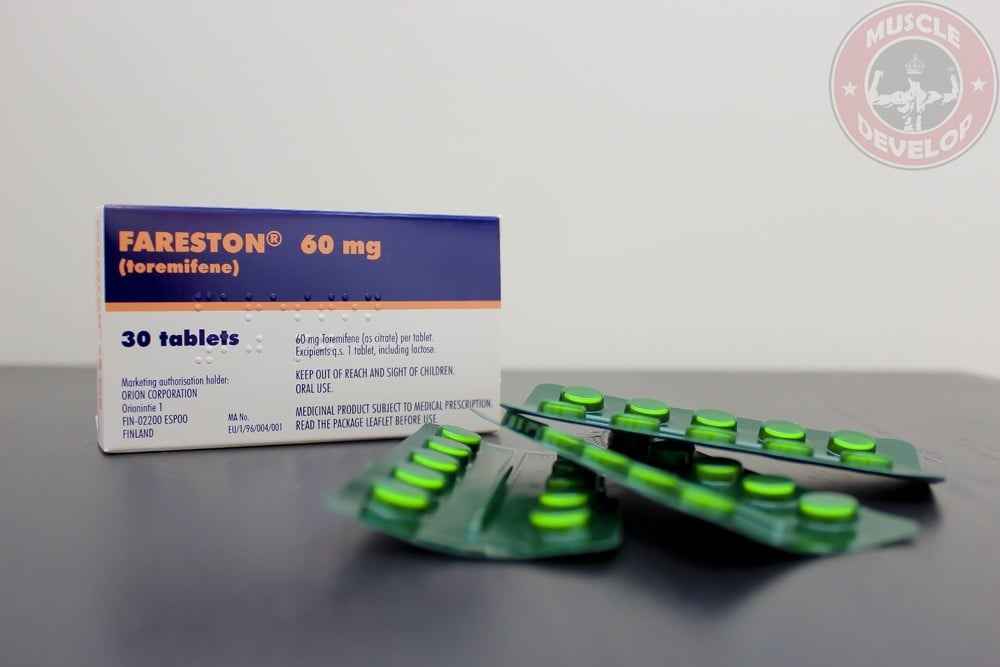
FARESTON 60 mg TABLETS

How to use FARESTON 60 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fareston 60 mg Tablets
toremifene
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Fareston and what is it used for
- What you need to know before you take Fareston
- How to take Fareston
- Possible side effects
5 Storage of Fareston
- Contents of the pack and further information
1. What is Fareston and what is it used for
Fareston contains the active substance toremifene, an anti-estrogen. Fareston is indicated for the treatment of certain types of breast tumors in women after menopause.
2. What you need to know before you take Fareston
Do not take Fareston
- if you are allergic to toremifene or any of the other ingredients of this medicine (listed in section 6).
- if you have a thickening of the lining of the uterus (endometrium)
- if you have severe liver problems
- if you have had a heart condition or have had any condition that affects the heart's electrical activity (electrocardiogram or ECG)
- if you have an imbalance of salts in the blood, especially low levels of potassium in the blood (hypokalemia) that are not corrected by treatment
- if you have a very slow heart rate (bradycardia)
- if you have a weak heart (heart failure)
- if you have a history of heart rhythm disorders (arrhythmias)
- if you are taking other medicines that may affect your heart (see section 2 "Taking Fareston with other medicines").
This is because Fareston can affect your heart by causing a delay in the electrical signals in your heart (prolongation of the QT interval).
Warnings and precautions
Talk to your doctor or pharmacist before starting Fareston.
- if you have unstable diabetes
- if your quality of life is severely impaired
- if you have previously had episodes of blood clots in the veins, such as in the lungs (pulmonary embolism) or in the veins of the legs (deep vein thrombosis).
- if you have heart rhythm disorders when taking Fareston. Your doctor may recommend that you stop taking Fareston and undergo a medical test to check how your heart is working (ECG, electrocardiogram), (see section 2 "Do not take Fareston")
- if you have any heart condition, including chest pain (angina)
- if the cancer has spread to the bones (bone metastasis) as the calcium level in the blood may increase at the start of treatment with Fareston. Your doctor will perform regular checks
- if your doctor has told you that you have an intolerance to certain sugars, such as lactose (see section 2 "Fareston contains lactose")
You should have gynecological examinations at the start of treatment with Fareston and at least once a year while you are taking Fareston. Your doctor will perform regular checks if you have high blood pressure, diabetes, have undergone hormone replacement therapy, or if you are obese (BMI over 30).
Other medicines and Fareston
Tell your doctor if you are taking, have recently taken, or might take any other medicines. It may be necessary to adjust the dose of one of them while you are taking Fareston. In particular, tell your doctor if you are taking any of the following medicines:
- thiazide diuretics
- medicines that prevent blood clotting, such as warfarin
- medicines used to treat epilepsy, such as carbamazepine, phenytoin, phenobarbital
- medicines used to treat fungal infections, such as ketoconazole, itraconazole, voriconazole, posaconazole
- medicines used to treat bacterial infections (antibiotics) such as erythromycin, clarithromycin, and telithromycin
- medicines used to treat viral infections such as ritonavir and nelfinavir
Do not take Fareston with the following medicines as there is a risk of altering your heart rhythm (see section 2 "Do not take Fareston"):
- medicines used to treat abnormal heart rhythms (anti-arrhythmics); such as quinidine, hydroquinidine, disopyramide, amiodarone, sotalol, dofetilide, ibutilide,
- medicines used to treat mental or behavioral disorders (neuroleptics) such as phenothiazines, pimozide, sertindole, haloperidol, sultopride,
- medicines for treating infections (antimicrobials) such as moxifloxacin, erythromycin (by infusion), pentamidine, and antimalarials (especially halofantrine)
- certain medicines for treating allergies; such as terfenadine, astemizole, mizolastine
- others (cisapride, intravenous vincamine, bepridil, diphemanil).
If you are admitted to hospital or prescribed a new medicine, tell your doctor that you are taking Fareston.
Pregnancy and breastfeeding
Do not take Fareston during pregnancy or breastfeeding.
Driving and using machines
Fareston has no influence on the ability to drive and use machines.
Fareston contains lactose
It contains 28.5 mg of lactose (as monohydrate) per tablet. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine.
Other excipients
This medicine contains less than 1 mmol of sodium (23 mg) per tablet; this is, essentially "sodium-free".
3. How to take Fareston
Follow exactly the instructions of administration of this medicine given to you by your doctor. If you are in doubt, consult your doctor or pharmacist again. The recommended dose is one 60 mg tablet per day, taken orally. Fareston can be taken with or without food.
If you take more Fareston than you should
Contact your doctor, pharmacist, or the nearest hospital immediately. The symptoms of overdose may be dizziness and headache.
If you forget to take Fareston
If you forget to take a dose, take the next tablet as usual and continue the treatment as recommended. Do not take a double dose to make up for forgotten doses. If you forget several doses, inform your doctor and follow their instructions.
If you stop taking Fareston
Treatment with Fareston should only be stopped when your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common (may affect more than 1 in 10 people)
- hot flashes, sweating
Common (may affect up to 1 in 10 people)
- fatigue, dizziness, depression
- nausea (feeling sick), vomiting
- skin rash, itching, swelling (edema)
- uterine bleeding, vaginal discharge of white fluid.
Uncommon (may affect up to 1 in 100 people)
- headache, sleep disturbances
- weight gain, constipation, loss of appetite
- increase in the size of the lining of the uterus (endometrial hyperplasia)
- blood clots, such as in the lungs (thromboembolic events)
- breathing difficulties.
Rare (may affect up to 1 in 1,000 people)
- feeling of spinning (vertigo)
- growth of the lining of the uterus (uterine polyps)
- increase in liver enzymes (increase in transaminases).
Very rare (may affect up to 1 in 10,000 people)
- changes in the lining of the uterus (endometrium), cancer of the lining of the uterus (endometrial cancer)
endometrial cancer
- hair loss (alopecia)
- clouding of the surface of the eye (transient corneal opacity)
- yellowing of the skin and the whites of the eyes (jaundice).
Frequency not known (cannot be estimated from the available data)
- low white blood cell count, which is important for fighting infection
(leukopenia)
- low red blood cell count (anemia)
- low platelet count (thrombocytopenia)
- inflammation of the liver (hepatitis)
You should contact your doctor immediately if you notice any of the following symptoms:
- swelling or unusual sensitivity in your calf
- unexplained breathing difficulties or sudden chest pain
- vaginal bleeding or changes in vaginal discharge.
Fareston causes certain abnormal changes in the electrical activity of the heart (electrocardiogram or ECG). See section 2 "Warnings and precautions".
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Annex V.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fareston
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Fareston
- The active substance is toremifene; each tablet contains 60 mg (as citrate).
- The other ingredients are corn starch, lactose monohydrate, povidone, sodium starch glycolate, microcrystalline cellulose, anhydrous colloidal silica, and magnesium stearate.
Appearance and packaging
The tablets are white, round, smooth, beveled, with TO 60 printed on one side.
30 and 100 tablets. Some pack sizes may not be marketed.
Marketing authorization holder
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
Manufacturer
Orion Corporation Orion Pharma
Joensuunkatu 7
FI-24100 Salo
Finland
You can obtain more information on this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Orion Corporation Tel: +358 10 4261 | Lithuania Orion Corporation Tel: +358 10 4261 |
Bulgaria Orion Pharma Poland Sp. z o.o. Tel: + 48 22 8 333 177 | Luxembourg/Luxemburg Orion Corporation Tel: +358 10 4261 |
Czech Republic Orion Corporation Tel: +358 10 4261 | Hungary Orion Corporation Tel: +358 10 4261 |
Denmark Orion Corporation Tel: +358 10 4261 | Malta Orion Corporation Tel: +358 10 4261 |
Germany Orion Corporation Tel: +358 10 4261 | Netherlands Orion Corporation Tel: +358 10 4261 |
Estonia Orion Corporation Tel: +358 10 4261 | Norway Orion Corporation Tel: +358 10 4261 |
Greece Orion Corporation Tel: +358 10 4261 | Austria Orion Corporation Tel: +358 10 4261 |
Spain Orion Corporation Tel: +358 10 4261 | Poland Orion Corporation Tel: +358 10 4261 |
France Orion Pharma Tel: +33 (0) 1 85 18 00 00 | Portugal Orion Corporation Tel: +358 10 4261 |
Croatia Orion Corporation Tel: +358 10 4261 | Romania Orion Pharma Poland Sp. z o.o. Tel: + 48 22 8 333 177 |
Ireland Orion Corporation Tel: +358 10 4261 | Slovenia Orion Corporation Tel: +358 10 4261 |
Iceland Orion Corporation Tel: +358 10 4261 | Slovakia Orion Corporation Tel: +358 10 4261 |
Italy Orion Pharma S.r.l. Tel: + 39 02 67876111 | Finland Orion Corporation Tel: +358 10 4261 |
Cyprus Orion Corporation Tel: +358 10 4261 | Sweden Orion Pharma AB Tel: +46 8 623 6440 |
Latvia Orion Corporation Tel: +358 10 4261 | United Kingdom (Northern Ireland) Orion Corporation Tel: + 358 10 4261 |
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FARESTON 60 mg TABLETSDosage form: TABLET, 60 mgActive substance: toremifeneManufacturer: Orion CorporationPrescription requiredDosage form: INJECTABLE, 250 mg/5 mlActive substance: fulvestrantManufacturer: Bexal Farmaceutica S.A.Prescription requiredDosage form: INJECTABLE, 250 mgActive substance: fulvestrantManufacturer: Ever Valinject GmbhPrescription required
Online doctors for FARESTON 60 mg TABLETS
Discuss questions about FARESTON 60 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













