
EVKEEZA 150 mg/mL concentrate for infusion solution

How to use EVKEEZA 150 mg/mL concentrate for infusion solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
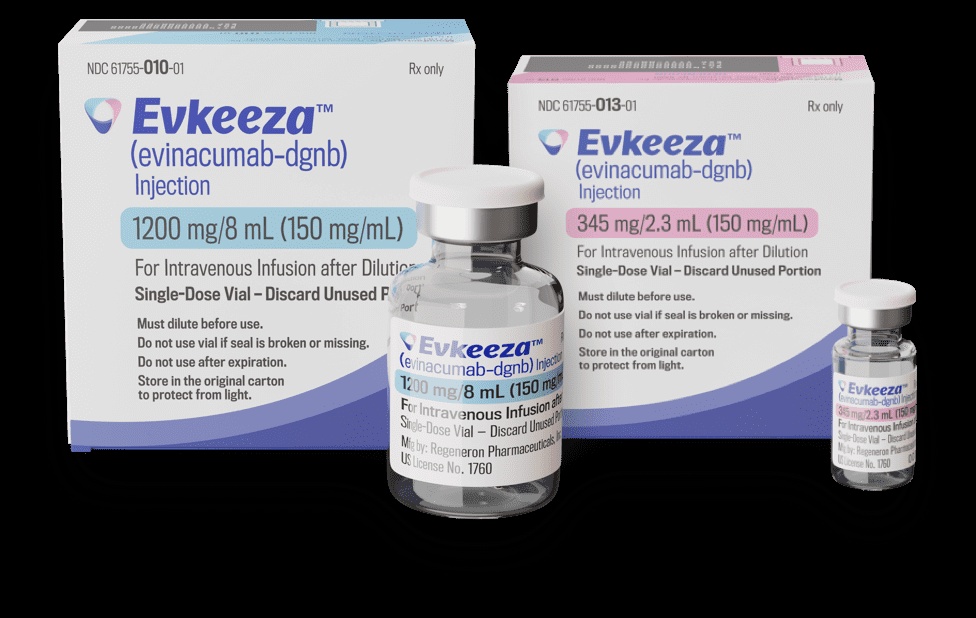
Evkeeza 150 mg/ml concentrate for solution for infusion
evinacumab
This medicine is subject to additional monitoring, which will allow for quicker identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Evkeeza and what is it used for
- What you need to know before you are given Evkeeza
- How Evkeeza is given
- Possible side effects
- Storage of Evkeeza
- Contents of the pack and other information
1. What is Evkeeza and what is it used for
What is Evkeeza
Evkeeza contains the active substance evinacumab. It is a type of medicine called a ‘monoclonal antibody’. Monoclonal antibodies are proteins that attach to other substances in the body.
What Evkeeza is used for
Evkeeza is used to treat adults and children from 6 months of age with very high cholesterol caused by a condition called homozygous familial hypercholesterolaemia. Evkeeza is used together with a low-fat diet and other medicines to lower cholesterol levels.
Homozygous familial hypercholesterolaemia is inherited and is usually passed on from both parents.
People with this condition have extremely high levels of LDL cholesterol (‘bad’ cholesterol) from birth. Such high levels can cause heart attacks, heart valve disease, or other problems at a young age.
How Evkeeza works
Evinacumab, the active substance in Evkeeza, attaches to a protein in the body called ANGPTL3 and blocks its effects. ANGPTL3 is involved in controlling the production of cholesterol, and blocking its effect reduces the production of cholesterol. In this way, Evkeeza can lower the levels of LDL cholesterol in the blood and prevent problems caused by high levels of LDL cholesterol.
2. What you need to know before you are given Evkeeza
You should not be given Evkeeza if:
- if you are allergic to evinacumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or nurse before you are given Evkeeza.
Be aware of serious side effects
Evkeeza can cause serious allergic reactions.
- Tell your doctor or nurse immediately if you have any symptoms of a serious allergic reaction. The symptoms are listed in ‘Serious side effects’ in section 4.
Children
Evkeeza is not recommended for children under 6 months of age because there is not enough information on its use in this group of patients.
Other medicines and Evkeeza
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Pregnancy and contraception
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
- Evkeeza may harm your unborn baby.
- Tell your doctor immediately if you become pregnant while being treated with Evkeeza.
If you can become pregnant, you must use effective contraceptive methods to prevent this.
- use effective contraceptive methods while being treated with Evkeeza and
- use effective contraceptive methods for at least 5 months after the last administration of Evkeeza.
Talk to your doctor about the best contraceptive method for you during this time.
Breast-feeding
- If you are breast-feeding or plan to breast-feed, consult your doctor before you are given this medicine.
- It is not known if Evkeeza passes into breast milk.
Driving and using machines
Evkeeza may make you feel dizzy and tired, and may affect your ability to ride a bicycle, drive, or use tools or machines. If you feel affected, do not ride a bicycle, do not drive, or use machines, and tell your doctor (see section 4).
Evkeeza contains proline
This medicine contains 30 mg of proline in each ml. Proline may be harmful for patients with hyperprolinemia, a rare genetic disorder in which proline accumulates in the body. If you (or your child) have hyperprolinemia, do not use this medicine unless your doctor has recommended it.
Evkeeza contains polysorbate 80
This medicine contains 1 mg of polysorbate 80 in each ml. Polysorbates may cause allergic reactions. Tell your doctor if you (or your child) have any known allergies.
3. How Evkeeza is given
How much Evkeeza is given
Your doctor will calculate the amount of medicine you should be given. The amount will depend on your body weight.
- The recommended dose is 15 milligrams per kilogram of body weight.
- You will be given the medicine approximately once a month.
How Evkeeza is given
Evkeeza is usually given by a doctor or nurse. It is given as an infusion into a vein (‘intravenous infusion’) over 60 minutes.
If you miss a dose of Evkeeza
If you have missed an appointment to receive Evkeeza, talk to your doctor or nurse as soon as possible.
If you have any other questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Reporting of side effects
Serious allergic reactions(uncommon: may affect up to 1 in 100 people)
Tell your doctor or nurse immediately if you experience any of the following symptoms of a serious allergic reaction (anaphylactic reaction). The infusion will be stopped immediately and you may need to take other medicines to control the reaction:
- swelling, mainly of the lips, tongue, or throat, which may make it difficult to swallow or breathe
- breathing problems or wheezing
- feeling dizzy or fainting
- rash, hives
- itching.
Other side effects
Talk to your doctor or nurse if you experience any of the following side effects:
Very common(may affect more than 1 in 10 people)
- common cold symptoms, such as runny nose (nasopharyngitis).
Common(may affect up to 1 in 10 people)
- feeling dizzy
- sore throat or infection of the nasal passages (upper respiratory tract infection)
- feeling sick (nausea)
- stomach pain
- constipation
- back pain
- pain in hands or feet (limb pain)
- flu-like symptoms
- feeling tired or weak (asthenia)
- infusion reaction, such as itching at the infusion site.
Other side effects in children from 5 to 11 years of age
Very common(may affect more than 1 in 10 people)
- feeling tired (fatigue).
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Evkeeza
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze. Do not shake.
Store in the original package to protect from light.
Do not use this medicine if you notice it is cloudy, has changed colour, or contains visible particles.
Do not keep any unused portion of the infusion solution for reuse. Any unused portion of the infusion solution must not be reused and must be disposed of in accordance with local requirements.
6. Contents of the pack and other information
What Evkeeza contains
- The active substance is evinacumab.
Each ml of concentrate for solution for infusion contains 150 mg of evinacumab.
Each vial contains 345 mg of evinacumab in 2.3 ml of concentrate or 1,200 mg of evinacumab in 8 ml of concentrate.
- The other ingredients are proline, arginine hydrochloride, histidine hydrochloride monohydrate, polysorbate 80, histidine, and water for injections.
Appearance and package contents
Evkeeza concentrate for solution for infusion is a clear to slightly opalescent, colourless to pale yellow solution.
It is available in packs of 1 vial of 2.3 ml concentrate or 1 vial of 8 ml concentrate.
Marketing authorisation holder
Ultragenyx Germany GmbH
Rahel-Hirsch-Str. 10
10557 Berlin
Germany
Manufacturer
Ultragenyx Netherlands B.V.
Evert van de Beekstraat 1, Unit 104
1118 CL Schiphol
Netherlands
You can request more information about this medicine from the local representative of the marketing authorisation holder:
BE, BG, CZ, DK, DE, EE, ES, HR, IE, IS, IT, CY, LI, LV, LT, LU, HU, MT, NL, NO, AT, PL, PT, RO, SI, SK, FI, SE
Ultragenyx Germany GmbH, DE
Tel: +49 30 20179810
EL
Medison Pharma Greece Single Member Societe Anonyme, EL
Tel: +30 210 0100 188
FR
Ultragenyx France SAS, FR
Tel: +33 1 85 65 37 61 or 0800 917 924 (free phone number)
Date of last revision of this leaflet:
This medicine has been authorised under ‘exceptional circumstances’. This means that due to the rarity of the disease, it has not been possible to obtain complete information on this medicine. The European Medicines Agency will review any new information on this medicine that becomes available every year, and this leaflet will be updated as necessary.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
This information is intended only for healthcare professionals:
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the product should be clearly recorded.
Instructions for use
Preparation of the solution
Evkeeza is supplied as a single-use vial only. During preparation and reconstitution, a strictly aseptic technique must be used.
- Visually inspect the medicine for cloudiness, change in colour, or particles before administration.
- Discard the vial if the solution is cloudy or has changed colour or contains particles.
- Do not shake the vial.
- Withdraw the required volume of evinacumab from the vial or vials based on the patient’s body weight and transfer it to an intravenous infusion bag containing sodium chloride 9 mg/ml (0.9%) or dextrose 50 mg/ml (5%) for infusion. Mix the diluted solution by gently inverting.
- The final concentration of the diluted solution should be between 0.5 mg/ml and 20 mg/ml.
- Do not freeze or shake the solution.
- Discard any unused portion remaining in the vial.
Disposal of unused medicine and all materials that have come into contact with it should be done in accordance with local regulations.
After dilution
Once prepared, the diluted solution should be administered immediately. If the diluted solution is not administered immediately, it can be stored temporarily:
- refrigerated between 2°C and 8°C for no more than 24 hours from the time of preparation of the infusion to the end of the infusion
or
- at room temperature up to 25°C for no more than 6 hours from the time of preparation of the infusion to the end of the infusion.
Administration
- If refrigerated, allow the solution to reach room temperature (up to 25°C) before administration.
- Evinacumab should be administered over 60 minutes through an intravenous line containing a sterile filter, in-line or add-on, of 0.2 microns to 5 microns. Do not administer evinacumab as a bolus or rapid intravenous injection.
- Do not mix other medicines with evinacumab or administer it concomitantly through the same infusion line.
The infusion rate may be slowed, interrupted, or discontinued if the patient exhibits any signs of adverse reactions, including symptoms associated with infusion.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EVKEEZA 150 mg/mL concentrate for infusion solutionDosage form: TABLET, 10 mg ezetimibeActive substance: ezetimibeManufacturer: Organon Salud S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Kern Pharma S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Strides Pharma (Cyprus) LimitedPrescription required
Online doctors for EVKEEZA 150 mg/mL concentrate for infusion solution
Discuss questions about EVKEEZA 150 mg/mL concentrate for infusion solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











