
EPORATIO 5000 IU/0.5 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use EPORATIO 5000 IU/0.5 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Eporatio 1,000 UI/0.5 ml solution for injection in pre-filled syringe
Eporatio 2,000 UI/0.5 ml solution for injection in pre-filled syringe
Eporatio 3,000 UI/0.5 ml solution for injection in pre-filled syringe
Eporatio 4,000 UI/0.5 ml solution for injection in pre-filled syringe
Eporatio 5,000 UI/0.5 ml solution for injection in pre-filled syringe
Eporatio 10,000 UI/1 ml solution for injection in pre-filled syringe
Eporatio 20,000 UI/1 ml solution for injection in pre-filled syringe
Eporatio 30,000 UI/1 ml solution for injection in pre-filled syringe
epoetin zeta (epoetin theta)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Eporatio and what is it used for
- What you need to know before you use Eporatio
- How to use Eporatio
- Possible side effects
- Storage of Eporatio
- Contents of the pack and other information
- Information for self-injection
1. What is Eporatio and what is it used for
What is Eporatio
Eporatio contains the active substance epoetin zeta (epoetin theta), which is almost identical to erythropoietin, a natural hormone produced by the body. Epoetin zeta (epoetin theta) is a protein produced by biotechnology. It works in exactly the same way as erythropoietin. Erythropoietin is produced in the kidneys and stimulates the bone marrow to produce red blood cells. Red blood cells are very important for distributing oxygen throughout the body.
What is Eporatio used for
Eporatio is used for the symptomatic treatment of anemia and its symptoms (e.g., fatigue, weakness, and shortness of breath). Anemia occurs when the blood cells do not contain enough red blood cells. Treatment for anemia is given to adult patients with chronic kidney disease or adult patients with non-myeloid cancer (cancer that does not originate in the bone marrow), who are also receiving chemotherapy (cancer medication) at the same time.
2. What you need to know before you use Eporatio
Do not use Eporatio
- if you are allergic to epoetin zeta (epoetin theta), other epoetins, or any of the other ingredients of this medicine (listed in section 6);
- if you have uncontrolled high blood pressure.
Warnings and precautions
General
This medicine may not be suitable for the following patients. Please consult your doctor if you belong to any of these patient groups:
- patients with liver problems
- patients with pathological changes in their red blood cells (homocytic sickle cell anemia).
Before and during treatment with this medicine, your blood pressure should be closely monitored. If your blood pressure increases, your doctor may give you medication to lower your blood pressure. If you are already being treated with medication to lower your blood pressure, your doctor may increase the dose. It may also be necessary to reduce the dose of Eporatio or interrupt treatment with Eporatio for a short period.
Consult a doctor immediately if you experience sudden, severe headache, confusion, altered speech, impaired gait, seizures, or convulsions. These may be signs of very high blood pressure, even if your blood pressure is normally normal or low. It is necessary to treat them at the same time.
Your doctor will regularly perform blood tests to monitor various blood components and their levels. Additionally, iron levels in the blood must be monitored before and during treatment with this medicine. If your iron levels are too low, your doctor may also prescribe an iron medication for you.
If you feel tired and weak or experience fatigue, you should consult your doctor. These symptoms could indicate that treatment with this medicine is ineffective. Your doctor will check that there are no other causes of anemia and may perform blood tests or a bone marrow examination.
The healthcare professional treating you will record exactly which product you are using at all times. This can help provide more information about the safety of medicines like this.
Healthy individuals should not use Eporatio. Using this medicine by healthy individuals can excessively increase certain blood parameters and cause heart or blood vessel problems, potentially endangering life.
Severe skin reactions, such as Stevens-Johnson syndrome (SSJ) and toxic epidermal necrolysis (TEN), have been observed with the administration of epoetins. SSJ/TEN may initially appear as red, circular patches, often with central blisters on the trunk. Ulcers in the mouth, throat, nose, genitals, and eyes (eye irritation and swelling) may also appear. These severe skin reactions are often preceded by fever or flu-like symptoms. The skin rash can progress to widespread skin peeling and potentially fatal complications. If you present with a severe skin rash or any of these other skin symptoms, stop using Eporatio and contact your doctor or seek immediate medical attention.
Anemia caused by chronic kidney disease
If you are a patient with chronic kidney disease, your doctor will monitor that a certain blood parameter (hemoglobin) does not exceed the defined threshold. If blood parameters become too high, heart or vascular problems may occur, increasing the risk of death.
If you are a patient with chronic kidney disease and, in particular, if you do not respond adequately to Eporatio, your doctor will monitor your Eporatio dose, as repeated dose increases of Eporatio if you are not responding to treatment may increase the risk of heart or blood vessel problems and may increase the risk of myocardial infarction, stroke, and death.
If you have kidney blood vessel hardening (nephrosclerosis) but do not require dialysis treatment, your doctor will consider whether treatment is suitable for you. In this case, it cannot be excluded with absolute certainty that the progression of kidney function deterioration may be accelerated.
If you are on dialysis, medications that prevent blood clotting are used. If you are being treated with Eporatio, the dose of the anticoagulant medication may need to be increased. Otherwise, the increase in red blood cells can cause blockage of the arteriovenous fistula (an artificial connection between an artery and a vein that is surgically created in dialysis patients).
Anemia in cancer patients
If you are a cancer patient, you should know that this medicine can act as a growth factor for blood cells and, in some circumstances, may have a negative impact on cancer. Depending on the individual situation, a blood transfusion may be preferable. Please discuss this with your doctor.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age, as there is no data to demonstrate the safety and efficacy of this medicine in this age group.
Other medicines and Eporatio
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
The use of Eporatio in pregnant women has not been investigated. It is important that you inform your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant, as your doctor will decide whether it is advisable for you not to use this medicine.
It is not known whether the active substance of this medicine passes into breast milk. Your doctor will decide whether it is advisable for you not to use this medicine while breastfeeding.
Driving and using machines
This medicine does not affect your ability to drive or use machines.
Eporatio contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe; this is essentially "sodium-free".
3. How to use Eporatio
Your treatment with this medicine will be started by a doctor with experience in the aforementioned indications.
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is…
The dose of Eporatio (expressed in International Units or IU) will depend on your disease, body weight, and the route of injection administration (under the skin [subcutaneous injection] or into a vein [intravenous injection]). Your doctor will indicate the correct dose for you.
Anemia caused by chronic kidney disease
Injections can be administered under the skin or into a vein. Patients on hemodialysis normally receive the injection at the end of dialysis via the arteriovenous fistula. Patients who are not on dialysis treatment normally receive the injection under the skin. Your doctor will regularly perform blood tests and adjust the dose or suspend treatment if necessary. Hemoglobin values in the blood should not exceed 12 g/dl (7.45 mmol/l). Your doctor will use the lowest effective dose to control the symptoms of anemia. If you do not respond adequately to Eporatio, your doctor will monitor your dose and inform you if it is necessary to change the doses of Eporatio.
Treatment with Eporatio is divided into two parts:
- Correction of anemia
The initial dose for subcutaneous injections is 20 IU per kilogram of body weight, administered three times a week. If necessary, your doctor will increase the dose at monthly intervals.
The initial dose for intravenous injections is 40 IU per kilogram of body weight, administered three times a week. If necessary, your doctor will increase the dose at monthly intervals.
- Maintenance of red blood cell levels
Once an adequate number of red blood cells has been achieved, the maintenance dose required to maintain a constant number will be determined by your doctor.
In the case of subcutaneous injections, the weekly dose may be administered either as one injection per week or as three divided injections per week.
In the case of intravenous injections, the dose may be adjusted to two injections per week.
If you modify the frequency of dose administration, a dose adjustment may be necessary.
Treatment with Eporatio is normally long-term treatment.
The maximum dose should not exceed 700 IU per kilogram of body weight per week.
Anemia in cancer patients
Subcutaneous injections. The injection will be administered once a week. The initial dose is 20,000 IU. Your doctor will regularly perform blood tests and adjust the dose or suspend treatment if necessary. Hemoglobin values in the blood should not exceed 12 g/dl (7.45 mmol/l). You will normally receive Eporatio until one month after finishing chemotherapy.
The maximum dose should not exceed 60,000 IU.
How are the injections administered?
This medicine is administered by injection using a pre-filled syringe. The injection is administered either into a vein (intravenous injection) or into the tissue just under the skin (subcutaneous injection).
If you receive Eporatio treatment as a subcutaneous injection, your doctor may suggest that you learn to inject this medicine yourself. Your doctor or nurse will give you instructions on how to do this. Do not attempt to administer this medicine yourself without these instructions. Part of the information you need to use the pre-filled syringe can be found at the end of this leaflet (see section 7, "Information for self-injection"). However, for optimal treatment of your disease, close and constant cooperation with your doctor is required.
Each pre-filled syringe is for single use only.
If you use more Eporatio than you should
Do not increase the dose that your doctor has prescribed for you. If you think you have injected more Eporatio than you should, consult your doctor. It is unlikely to be serious. Even with very high levels in the blood, no symptoms of poisoning have been observed.
If you forget to use Eporatio
If you have forgotten an injection or have injected too little, tell your doctor. Do not use a double dose to make up for forgotten doses.
If you stop treatment with Eporatio
Before stopping treatment with this medicine, consult your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects:
- Very high blood pressure:
Consult a doctor immediately if you experience sudden, severe headache, confusion, altered speech, impaired gait, seizures, or convulsions. These may be signs of very high blood pressure (frequent in patients with chronic kidney disease, may affect up to 1 in 10 people), even if your blood pressure is normally normal or low. It is necessary to treat them at the same time.
- Allergic reactions:
Allergic reactions such as skin rash, itching over large areas of skin, severe allergic reactions with weakness, low blood pressure, difficulty breathing, and swelling of the face (frequency not known, cannot be estimated from the available data) have been reported. If you believe you have had this type of reaction, you must stop treatment with Eporatio and seek immediate medical attention.
- Severe skin reactions:
Severe skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been observed with the administration of epoetins. These reactions may appear as red, circular patches, often with central blisters on the trunk, skin peeling, and ulcers in the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms. Stop using Eporatio if you present with these symptoms and contact your doctor or seek immediate medical attention. See also section 2.
You may experience the following additional side effects:
Frequent (may affect up to 1 in 10 people)
- Headache;
- High blood pressure;
- Flu-like symptoms, such as fever, chills, feeling weak, fatigue;
- Skin reactions, such as rash, itching, or injection site reactions.
Frequent in patients with chronic kidney disease (may affect up to 1 in 10 people)
- Blood clot in the arteriovenous fistula in patients on dialysis.
Frequent in cancer patients (may affect up to 1 in 10 people)
- Joint pain.
Frequency not known in patients with chronic kidney disease (cannot be estimated from the available data)
- Cases of a disease called pure red cell aplasia (PRCA) have been reported.
PRCA means that the body stops or reduces the production of red blood cells, causing severe anemia. If your doctor suspects or confirms that you have this disease, you should not be treated with Eporatio or any other epoetin.
Frequency not known in cancer patients (cannot be estimated from the available data)
- Thromboembolic episodes, such as blood clots.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Eporatio
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton and on the pre-filled syringe after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the pre-filled syringe in the outer carton to protect it from light.
You may take Eporatio out of the refrigerator and store it at a temperature not above 25°C for a period of up to 7 days without exceeding the expiry date. Once it is taken out of the refrigerator, the medicine must be used within this time period or discarded.
Do not use this medicine if you notice turbidity or particles in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Eporatio
- The active ingredient is epoetin zeta (epoetin theta).
Eporatio 1,000 UI/0.5 ml: A pre-filled syringe contains 1,000 international units (IU) (8.3 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 2,000 international units (IU) (16.7 micrograms) per ml.
Eporatio 2,000 UI/0.5 ml: A pre-filled syringe contains 2,000 international units (IU) (16.7 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 4,000 international units (IU) (33.3 micrograms) per ml.
Eporatio 3,000 UI/0.5 ml: A pre-filled syringe contains 3,000 international units (IU) (25 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 6,000 international units (IU) (50 micrograms) per ml.
Eporatio 4,000 UI/0.5 ml: A pre-filled syringe contains 4,000 international units (IU) (33.3 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 8,000 international units (IU) (66.7 micrograms) per ml.
Eporatio 5,000 UI/0.5 ml: A pre-filled syringe contains 5,000 international units (IU) (41.7 micrograms) of epoetin zeta (epoetin theta) in 0.5 ml of injectable solution, equivalent to 10,000 international units (IU) (83.3 micrograms) per ml.
Eporatio 10,000 UI/1 ml: A pre-filled syringe contains 10,000 international units (IU) (83.3 micrograms) of epoetin zeta (epoetin theta) in 1 ml of injectable solution, equivalent to 10,000 international units (IU) (83.3 micrograms) per ml.
Eporatio 20,000 UI/1 ml: A pre-filled syringe contains 20,000 international units (IU) (166.7 micrograms) of epoetin zeta (epoetin theta) in 1 ml of injectable solution, equivalent to 20,000 international units (IU) (166.7 micrograms) per ml.
Eporatio 30,000 UI/1 ml: A pre-filled syringe contains 30,000 international units (IU) (250 micrograms) of epoetin zeta (epoetin theta) in 1 ml of injectable solution, equivalent to 30,000 international units (IU) (250 micrograms) per ml.
- The other components are sodium dihydrogen phosphate dihydrate, sodium chloride, polysorbate 20, trometamol, hydrochloric acid (6M) (for pH adjustment), and water for injection.
Product Appearance and Container Contents
Eporatio is a clear and colorless injectable solution in a pre-filled syringe with a needle for injection.
Eporatio 1,000 UI/0.5 ml, Eporatio 2,000 UI/0.5 ml, Eporatio 3,000 UI/0.5 ml, Eporatio 4,000 UI/0.5 ml, and Eporatio 5,000 UI/0.5 ml: Each pre-filled syringe contains 0.5 ml of solution.
Packs of 6 pre-filled syringes; 6 pre-filled syringes with safety needle or 6 pre-filled syringes with safety device.
Eporatio 10,000 UI/1 ml, Eporatio 20,000 UI/1 ml, and Eporatio 30,000 UI/1 ml: Each pre-filled syringe contains 1 ml of solution. Packs of 1, 4, and 6 pre-filled syringes; 1, 4, and 6 pre-filled syringes with safety needle or 1, 4, and 6 pre-filled syringes with safety device.
Only some pack sizes may be marketed.
Marketing Authorization Holder
ratiopharm GmbH
Graf-Arco-Strasse 3
89079 Ulm
Germany
Manufacturer
Teva Biotech GmbH
Dornierstrasse 10
89079 Ulm
Germany
Merckle GmbH
Graf-Arco-Straße 3
89079 Ulm
Germany
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
Belgium Teva Pharma Belgium N.V./S.A./AG Tel: +32 38207373 | Lithuania UAB Teva Baltics Tel: +370 52660203 |
| Luxembourg ratiopharm GmbH Germany Tel: +49 73140202 |
Czech Republic Teva Pharmaceuticals CR, s.r.o. Tel: +420 251007111 | Hungary Teva Gyógyszergyár Zrt. Tel: +36 12886400 |
Denmark Teva Denmark A/S Tlf: +45 44985511 | Malta Teva Pharmaceuticals Ireland Ireland Tel: +44 2075407117 |
Germany ratiopharm GmbH Tel: +49 73140202 | Netherlands Teva Nederland B.V. Tel: +31 8000228400 |
Estonia UAB Teva Baltics Eesti filiaal Tel: +372 6610801 | Norway Teva Norway AS Tlf: +47 66775590 |
Greece Specifar Α.Β.Ε.Ε. Τηλ: +30 2118805000 | Austria ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
Spain Teva Pharma, S.L.U. Tel: +34 913873280 | Poland Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 223459300 |
France Teva Santé Tél: +33 155917800 | Portugal ratiopharm - Comércio e Indústria de Produtos Farmacêuticos, Lda. Tel: +351 214248000 |
Croatia Pliva Hrvatska d.o.o. Tel: +385 13720000 | Romania Teva Pharmaceuticals S.R.L. Tel: +40 212306524 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 2075407117 | Slovenia Pliva Ljubljana d.o.o. Tel: +386 15890390 |
Iceland Teva Pharma Iceland ehf. Sími: +354 5503300 | Slovakia TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 257267911 |
Italy Teva Italia S.r.l. Tel: +39 028917981 | Finland Teva Finland Oy Puh/Tel: +358 201805900 |
Cyprus Specifar Α.Β.Ε.Ε. Greece Τηλ: +30 2118805000 | Sweden Teva Sweden AB Tel: +46 42121100 |
Latvia UAB Teva Baltics filiale Latvija Tel: +371 67323666 | United Kingdom (Northern Ireland) Teva Pharmaceuticals Ireland Ireland Tel: +44 2075407117 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Information for Self-Administration
This section contains information on how to administer Eporatio to yourself under the skin. It is essential that you do not attempt to administer an injection without first receiving the necessary instructions from your doctor or nurse. If you are unsure about your ability to administer the injection or have any doubts, consult your doctor or nurse.
How to Administer Eporatio
It should be injected into the tissue just under the skin. This is known as a subcutaneous injection.
Equipment Needed for Administration
To administer the injection under the skin, you will need:
- a pre-filled syringe of Eporatio,
- an alcohol swab,
- a sterile gauze pad or swab,
- a puncture-proof container (plastic container provided by a hospital or pharmacy) to safely dispose of used syringes.
What to Do Before Administering the Injection
- Remove a blister pack containing a pre-filled syringe from the refrigerator.
- Open the blister pack and remove the pre-filled syringe and needle from the pack. Do not touch the pre-filled syringe by the plunger or the syringe cap.
- Check the expiration date on the pre-filled syringe label (EXP). Do not use it if the date is later than the last day of the month shown.
- Check the appearance of Eporatio. It should be a clear and colorless liquid. If there are particles inside or it is cloudy, do not use it.
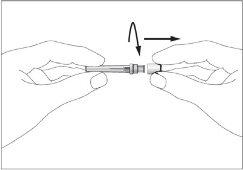
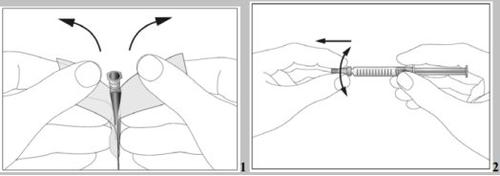
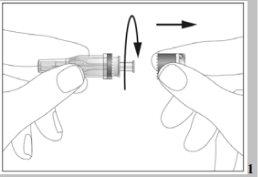
- There is a cap at the end of the needle. Break the security seal and remove the cap (see image 1).
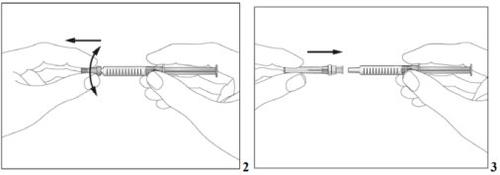
- Remove the cap from the pre-filled syringe (see image 2).
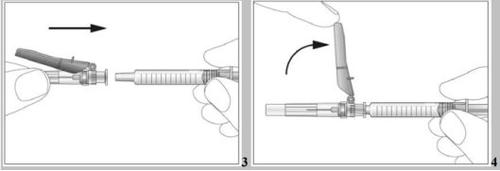
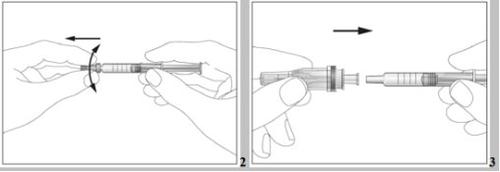
- Attach the needle to the pre-filled syringe (see image 3). Do not remove the syringe cap yet.
- To make the injection more comfortable, let the pre-filled syringe stand at room temperature (not above 25°C) for 30 minutes or gently hold the pre-filled syringe in your hands for a few minutes. Do notheat Eporatio in any other way (e.g., do not heat it in a microwave or in hot water).
- Do not remove the syringe cap until you are ready for the injection.
- Find a comfortable and well-lit place. Place everything you need within reach (the pre-filled syringe of Eporatio, the alcohol swab, a sterile gauze pad or swab, and a puncture-proof container).
- Wash your hands carefully.
How to Prepare for the Injection of Eporatio
Before administering Eporatio to yourself, do the following:
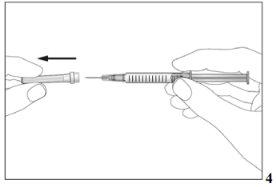
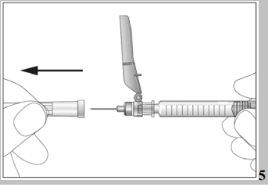
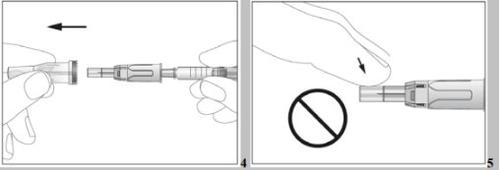
- Take the pre-filled syringe and carefully remove the protective cover from the needle without tilting it. Separate it as indicated in the images 4. Do not touch the needle or push the plunger.
- Small air bubbles may appear in the pre-filled syringe. If there are bubbles, gently tap the pre-filled syringe with your fingers until the bubbles rise to the top of the pre-filled syringe. With the pre-filled syringe pointing upwards, withdraw all the air from the pre-filled syringe by gently pushing the plunger upwards.
- The pre-filled syringe has a scale on the syringe body. Push the plunger to the number (IU) on the pre-filled syringe that corresponds to the dose of Eporatio prescribed by your doctor.
- Check again that the dose of Eporatio in the pre-filled syringe is correct.
- You can now use the pre-filled syringe.
Where to Inject
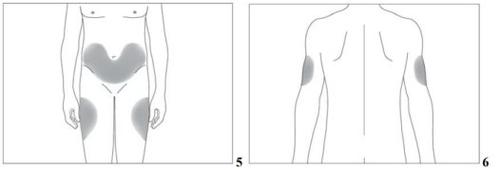
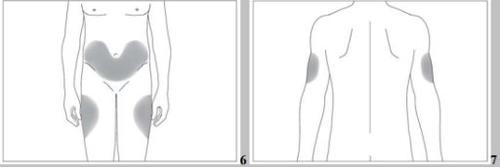
The most suitable places for injection are:
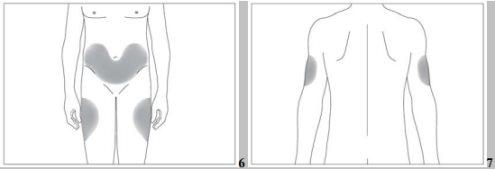
- the upper part of the thighs,
- the abdomen, except for the area around the navel (see the gray areas in image 5).
If someone helps you with the injection, you can also inject it into the back and lateral part of the arms (see the gray areas in image 6).
It is best to change the injection site every day to avoid the risk of pain appearing in any of the areas.
How to Inject
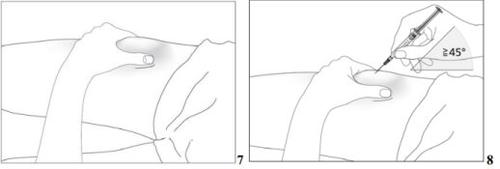
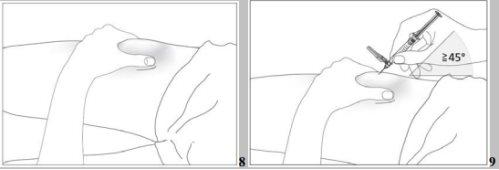
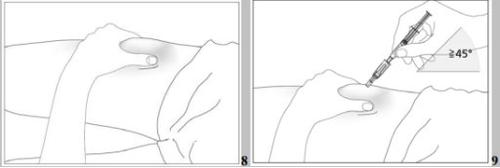
- Disinfect the injection site using an alcohol swab and pinch the skin between your thumb and index finger without squeezing (see image 7).
- Insert the needle completely into the skin as your doctor or nurse instructed you. The angle between the pre-filled syringe and the skin should not be too small (at least 45°, see image 8).
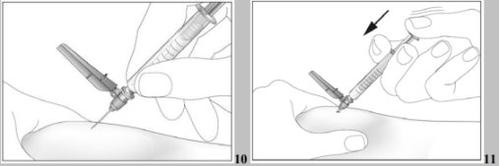
- Inject the liquid into the tissue slowly and regularly, always keeping the skin pinched.
- After injecting the liquid, withdraw the needle and remove it from the skin.
- Press the injection site with a sterile gauze pad or swab for several seconds.
- Use only one pre-filled syringe for a single injection. Do not use any remaining Eporatio from the pre-filled syringe.
Remember
If you have any problems, please ask for help or advice from your doctor or nurse.
How to Dispose of Used Syringes
- Do not put the protective cap back on used syringes.
- Place used syringes in a puncture-proof container and keep this container out of sight and reach of children.
- Dispose of the puncture-proof container according to the instructions of your doctor, pharmacist, or nurse.
- Never put used syringes in your household trash.
- Information for Self-Administration
This section contains information on how to administer Eporatio to yourself under the skin. It is essential that you do not attempt to administer an injection without first receiving the necessary instructions from your doctor or nurse. If you are unsure about your ability to administer the injection or have any doubts, consult your doctor or nurse.
How to Administer Eporatio
It should be injected into the tissue just under the skin. This is known as a subcutaneous injection.
Equipment Needed for Administration
To administer the injection under the skin, you will need:
- a pre-filled syringe of Eporatio,
- an alcohol swab,
- a sterile gauze pad or swab,
- a puncture-proof container (plastic container provided by a hospital or pharmacy) to safely dispose of used syringes.
What to Do Before Administering the Injection
- Remove a blister pack containing a pre-filled syringe from the refrigerator.
- Open the blister pack and remove the pre-filled syringe and needle from the pack. Do not touch the pre-filled syringe by the plunger or the syringe cap.
- Check the expiration date on the pre-filled syringe label (EXP). Do not use it if the date is later than the last day of the month shown.
- Check the appearance of Eporatio. It should be a clear and colorless liquid. If there are particles inside or it is cloudy, do not use it.
- There are flaps at the end of the needle bag. Open the needle bag by the flaps (see image 1).
- Remove the cap from the pre-filled syringe (see image 2).
- Attach the needle to the pre-filled syringe (see image 3). Do not remove the syringe cap yet.
- Move the safety protector away from the needle towards the syringe body. The safety protector will remain in the position you determine (see image 4).
- To make the injection more comfortable, let the pre-filled syringe stand at room temperature (not above 25°C) for 30 minutes or gently hold the pre-filled syringe in your hands for a few minutes. Do notheat Eporatio in any other way (e.g., do not heat it in a microwave or in hot water).
- Do not remove the syringe cap until you are ready for the injection.
- Find a comfortable and well-lit place. Place everything you need within reach (the pre-filled syringe of Eporatio, the alcohol swab, a sterile gauze pad or swab, and a puncture-proof container).
- Wash your hands carefully.
How to Prepare for the Injection of Eporatio
Before administering Eporatio to yourself, do the following:
- Take the pre-filled syringe and carefully remove the protective cover from the needle without tilting it. Separate it as indicated in image 5. Do not touch the needle or push the plunger.
- Small air bubbles may appear in the pre-filled syringe. If there are bubbles, gently tap the pre-filled syringe with your fingers until the bubbles rise to the top of the pre-filled syringe. With the pre-filled syringe pointing upwards, withdraw all the air from the pre-filled syringe by gently pushing the plunger upwards.
- The pre-filled syringe has a scale on the syringe body. Push the plunger to the number (IU) on the pre-filled syringe that corresponds to the dose of Eporatio prescribed by your doctor.
- Check again that the dose of Eporatio in the pre-filled syringe is correct.
- You can now use the pre-filled syringe.
Where to Inject
The most suitable places for injection are:
- the upper part of the thighs,
- the abdomen, except for the area around the navel (see the gray areas in image 6).
If someone helps you with the injection, you can also inject it into the back and lateral part of the arms (see the gray areas in image 7).
It is best to change the injection site every day to avoid the risk of pain appearing in any of the areas.
How to Inject
- Disinfect the injection site using an alcohol swab and pinch the skin between your thumb and index finger without squeezing (see image 8).
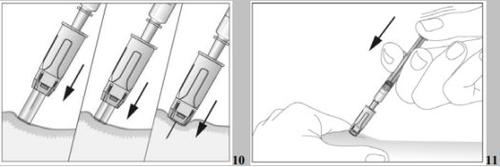
- Insert the needle completely into the skin as your doctor or nurse instructed you. The angle between the pre-filled syringe and the skin should not be too small (at least 45°, see images 9 and 10).
- Inject the liquid into the tissue slowly and regularly, always keeping the skin pinched (see image 11).
- After injecting the liquid, withdraw the needle and remove it from the skin.
- Press the injection site with a sterile gauze pad or swab for several seconds.
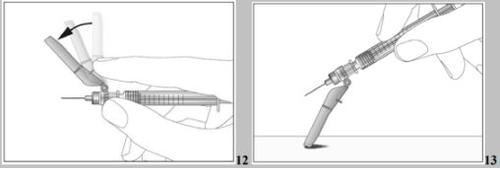
- Push the safety protector towards the needle (see image 12).
- Place the safety protector against a flat surface at an angle of approximately 45° (see image 13).
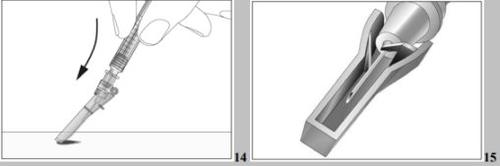
- Press the needle downwards with a firm and quick movement until a clear and audible click is produced (see image 14).
- Visually confirm that the needle is completely inserted into the safety protector.
- Use the locking system (see image 15).
- Use only one syringe for one injection. Do not use any remaining Eporatio from the syringe.
Remember
If you have any problems, please ask for help or advice from your doctor or nurse.
How to dispose of used syringes
- Deposit used syringes in a puncture-proof container and keep this container out of sight and reach of children.
- Dispose of the puncture-proof container according to the instructions of your doctor, pharmacist, or nurse.
- Never put the syringes you have used in your household trash can.
- Information for self-injection
This section contains information on how to administer Eporatio injections under the skin yourself. It is essential that you do not attempt to administer an injection without first receiving the necessary instructions from your doctor or nurse. If you are unsure about injecting yourself or have any doubts, consult your doctor or nurse.
How to administer Eporatio
It should be injected into the tissue just under the skin. This is known as a subcutaneous injection.
Equipment needed for administration
To administer the injection into the tissue just under the skin, you will need:
- a pre-filled Eporatio syringe,
- an alcohol swab,
- a sterile gauze pad or swab.
What to do before administering the injection
- Take a blister pack with a pre-filled syringe out of the refrigerator.
- Open the blister pack and remove the pre-filled syringe and needle from the pack. Do not handle the pre-filled syringe by the plunger or the syringe cap.
- Check the expiration date on the label of the pre-filled syringe (EXP). Do not use it if the date is later than the last day of the month shown.
- Check the appearance of Eporatio. It should be a clear, colorless liquid. If there are particles inside or it is cloudy, do not use it.
- There is a cap on the end of the needle. Break the security seal and remove the cap (see image 1)
- Remove the cap from the pre-filled syringe (see image 2)
- Attach the needle to the syringe (see image 3). Do not remove the syringe cap yet.
- For a more comfortable injection, let the syringe stand at room temperature (not above 25°C) for 30 minutes or gently hold the pre-filled syringe in your hands for a few minutes. Do notheat Eporatio in any other way (for example, do not heat it in a microwave or in hot water)
- Do not remove the syringe cap until you are ready for the injection
- Find a comfortable and well-lit place. Place everything you need within reach (the pre-filled Eporatio syringe, the alcohol swab, and a sterile gauze pad or swab).
- Wash your hands carefully
How to prepare for the Eporatio injection
Before administering the Eporatio injection yourself, do the following:
- Take the syringe and carefully remove the protective cover from the needle without tilting it. Separate it as indicated in images 4. The needle is surrounded by a retractable needle protector. Do not touch the needle or the needle protector, or push the plunger (see image 5).
- Small air bubbles may appear in the pre-filled syringe. If there are bubbles, gently tap the syringe with your fingers until the bubbles rise to the top of the syringe. With the syringe pointing upwards, remove all the air from the syringe by gently pushing the plunger upwards.
- The syringe has a scale on the body of the syringe. Push the plunger until the number (UI) on the syringe corresponds to the prescribed dose of Eporatio by your doctor.
- Check again that the dose of Eporatio in the syringe is correct.
- You can now use the pre-filled syringe.
Where to put the injection
The most suitable places for injection are:
- the upper part of the thighs,
- the abdomen, except the area around the navel (see the gray areas in image 6).
If someone helps you with the injection, you can also inject it into the back and side of the arms (see the gray areas in image 7)
It is best to change the injection site every day to avoid the risk of pain in any of the areas.
How to inject
- Disinfect the injection site using an alcohol swab and pinch the skin between your thumb and index finger, without squeezing (see image 8).
- Insert the protected needle completely into the skin, without hesitation and with a continuous motion, as indicated by your doctor or nurse. The angle between the syringe and the skin should not be too small (at least 45°, see image 9). The needle protector will retract completely when you insert the needle into the skin (see image 10).
- Inject the liquid into the tissue slowly and regularly, always keeping the skin pinched (see image 11).
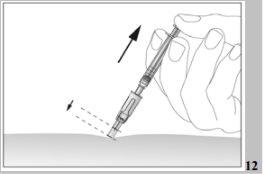
- After injecting the liquid, remove the needle and pull it out of the skin. The needle will be automatically protected and locked to prevent you from pricking yourself (see image 12),
- Press the injection site with a sterile gauze pad or swab for several seconds.
- Use only one syringe for one injection. Do not use any remaining Eporatio from the syringe.
Remember
If you have any problems, please ask for help or advice from your doctor or nurse.
How to dispose of used syringes
The safety device prevents puncture injuries after use, so no special precautions are required for disposal. Dispose of the syringes with the safety device according to the instructions of your doctor, pharmacist, or nurse.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EPORATIO 5000 IU/0.5 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 20000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 20,000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40,000 IU/ml of epoetin alfaActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription required
Online doctors for EPORATIO 5000 IU/0.5 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about EPORATIO 5000 IU/0.5 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















