DORZOLAMIDE/TIMOLOL STULLN 20 mg/mL + 5 mg/mL EYE DROPS SOLUTION


How to use DORZOLAMIDE/TIMOLOL STULLN 20 mg/mL + 5 mg/mL EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Dorzolamida/Timolol Stulln 20 mg/ml + 5 mg/ml eye drops, solution
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Dorzolamida/Timolol Stulln is and what it is used for
- What you need to know before you use Dorzolamida/Timolol Stulln
- How to use Dorzolamida/Timolol Stulln
- Possible side effects
- Storage of Dorzolamida/Timolol Stulln
- Contents of the pack and other information
1. What Dorzolamida/Timolol Stulln is and what it is used for
Dorzolamida/Timolol Stulln contains two active substances: dorzolamida and timolol.
- Dorzolamida belongs to a group of medicines called "carbonic anhydrase inhibitors".
- Timolol belongs to a group of medicines called "beta-blockers".
These medicines reduce pressure in the eye in different ways.
Dorzolamida/Timolol Stulln is prescribed to reduce high pressure in the eye in the treatment of glaucoma when the use of a single beta-blocker eye drop is not appropriate.
2. What you need to know before you use Dorzolamida/Timolol Stulln
Do not useDorzolamida/Timolol Stulln
- if you are allergic to dorzolamida hydrochloride, timolol maleate or any of the other ingredients of this medicine (listed in section 6).
- if you have now or have had in the past respiratory problems, such as asthma or severe chronic obstructive pulmonary disease (severe lung disease that may cause wheezing, difficulty breathing and/or persistent cough over a long period of time).
- if you have a slow heart rate, heart failure or irregular heart rhythm (irregular heartbeats).
- if you suffer from severe kidney or urinary tract problems, or have a history of kidney stones.
- if you have excess acidity of the blood caused by an accumulation of chlorides in the blood (hyperchloremic acidosis).
If you are not sure if you should use this medicine, consult your doctor or pharmacist.
Warnings and precautions
Consult your doctor before starting to use this medicine if you have or have had in the past:
- coronary heart disease (symptoms may include chest pain or tightness, difficulty breathing or shortness of breath), heart failure, low blood pressure.
- irregular heart rhythms such as slow heart rate.
- respiratory problems, asthma or chronic obstructive pulmonary disease.
- poor circulation (such as Raynaud's disease or Raynaud's syndrome).
- diabetes, as timolol may mask signs and symptoms of low blood sugar.
- Overactivity of the thyroid gland, as timolol may mask signs and symptoms.
Tell your doctor before having surgery that you are using this medicine, as timolol may affect the effects of some medicines used during anesthesia.
Also, tell your doctor about any allergy or allergic reactions including hives, swelling of the face, lips, tongue and/or throat that may cause difficulty breathing or swallowing.
Tell your doctor if you have muscle weakness or if you have been diagnosed with severe myasthenia gravis.
If you develop any other eye irritation or new eye problems, such as redness of the eyes or swelling of the eyelids, consult your doctor immediately.
If you suspect that this medicine is causing an allergic reaction or hypersensitivity (for example, skin rash, severe skin reaction or redness and itching of the eyes), stop using this medicine and consult your doctor immediately.
Tell your doctor if an eye infection occurs, if you suffer an eye injury, if you undergo eye surgery or if you develop a reaction that includes new symptoms or worsening of existing ones.
When this medicine is instilled in the eye, it may affect the whole body.
If you wear soft contact lenses, you should consult your doctor before using this medicine.
Use in children
There is limited experience with dorzolamida/timolol eye drops, solution (preservative-containing formulation) in infants and children.
Use in elderly patients
In studies with dorzolamida/timolol eye drops, solution (preservative-containing formulation), the effects were similar in both elderly and younger patients.
Use in patients with liver impairment
Tell your doctor if you have or have had liver problems.
Use in athletes:
This medicine contains timolol, which may produce a positive result in doping tests.
Use ofDorzolamida/Timolol Stullnwith other medicines
This medicine may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or plan to use medicines to lower blood pressure, heart medicines or medicines to treat diabetes. Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This is particularly important if you are:
- taking medicines to lower blood pressure or to treat heart conditions such as calcium channel blockers, beta-blockers or digoxin.
- taking medicines to treat irregular heart rhythms such as calcium channel blockers, beta-blockers or digoxin.
- using another eye drop that contains beta-blockers.
- taking another carbonic anhydrase inhibitor such as acetazolamide.
- taking monoamine oxidase inhibitors (MAOIs).
- taking a parasympathomimetic drug that may have been prescribed to help you urinate. Parasympathomimetics are also a type of medicine that is sometimes used to help restore normal movement through the intestine.
- taking narcotics, such as morphine used to treat moderate to severe pain.
- taking medicines to treat diabetes.
- taking antidepressants known as fluoxetine and paroxetine.
- taking a sulfonamide.
- taking quinidine (used to treat heart disorders and some types of malaria).
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine.
Pregnancy
Do not use this medicine if you are pregnant, unless your doctor considers it necessary.
Breastfeeding
Do not use this medicine if you are breastfeeding your child. Timolol may pass into breast milk. Ask your doctor for advice before taking any medicine during breastfeeding.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed. There are side effects associated with this medicine, such as blurred vision, that may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
This medicine contains benzalkonium chloride
This medicine contains 0.375 mg of benzalkonium chloride in 5 ml and 0.75 mg of benzalkonium chloride in 10 ml, equivalent to 0.075 mg/ml.
Benzalkonium chloride may be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medicine and wait 15 minutes before putting them back.
Benzalkonium chloride may cause eye irritation, especially if you have dry eyes or other corneal diseases (diseases of the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, stinging or pain in the eye after using this medicine.
3. How to use Dorzolamida/Timolol Stulln
Follow exactly the instructions of administration of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again. The appropriate dosage and duration of treatment will be determined by your doctor.
The recommended dose is one drop in the affected eye(s) in the morning and at night.
If you are using this medicine at the same time as another eye drop, the drops should be instilled at least 10 minutes apart.
Do not change the dose of the medicine without consulting your doctor.
Do not let the tip of the multidose container touch the eyes or the areas surrounding them. It may be contaminated with bacteria that can cause eye infections that can lead to serious damage to the eyes, including loss of vision. To avoid possible contamination of the multidose container, wash your hands before using this medicine and avoid touching the tip of the multidose container to any surface. If you think your medicine may be contaminated, or if you develop an eye infection, consult your doctor immediately about continuing to use this bottle.
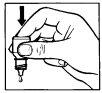
Instructions for use
If you have difficulty administering the drops, seek help from a family member or caregiver.
|
|
|
Figure 1 | Figure 2 | Figure 3 |
- First, wash your hands and unscrew the cap of the bottle.
- Tilt your head back and gently pull the lower eyelid down to form a pocket between the eyelid and the eye. (Figure 1)
- Press the bottle to release one drop into your eye. (Figure 2)
Do not touch your eye or eyelid with the tip of the dropper.
- After using this medicine, close your eye and press the inner corner of your eye with your finger for about two minutes (Figure 3). This helps prevent the medicine from reaching the rest of the body.
- Repeat steps 2 to 4 with the other eye if your doctor has told you to do so. Replace the cap and close the container tightly after use.
If you use moreDorzolamida/Timolol Stullnthan you should
If you instill too many drops in the eye or swallow some of the contents of the bottle, among other effects, you may feel dizziness, have difficulty breathing or notice that your heart beats more slowly. Contact your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken.
If you forget to useDorzolamida/Timolol Stulln
It is important to use this medicine as your doctor has prescribed.
If you forget to apply a dose, you should apply it as soon as possible. However, if it is almost time for the next dose, do not apply the missed dose and continue with the scheduled dosing program as usual.
Do not use a double dose to make up for missed doses.
If you stop usingDorzolamida/Timolol Stulln
If you want to stop treatment with this medicine, consult your doctor first.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Usually, you can continue using the drops, unless the effects are severe. If you are concerned, consult your doctor or pharmacist. Do not stop using this medicine without talking to your doctor.
Generalized allergic reactions may occur, including swelling under the skin in areas such as the face and limbs, and may obstruct the airways, causing difficulty swallowing or breathing, hives or skin rash with itching, generalized and localized rash, itching, severe allergic reaction that can be life-threatening.
The following adverse reactions have been reported with dorzolamida/timolol eye drops, solution (preservative-containing formulation) or with one of its components during clinical trials or during post-marketing experience:
Very common(may affect more than 1 in 10 people)
Burning and stinging in the eyes, alteration of taste.
Common(may affect up to 1 in 10 people)
Redness in and around the eye(s), tearing or itching in the eye(s), corneal erosion (damage to the front layer of the eyeball), inflammation and/or irritation in and around the eye(s), foreign body sensation in the eye (feeling that something is in the eye), decreased sensitivity of the cornea (not feeling that something is in the eye and not feeling pain), eye pain, dry eyes, blurred vision, headache, sinusitis (feeling of tension or congestion in the nose), nausea, weakness/fatigue and tiredness.
Uncommon(may affect up to 1 in 100 people)
Dizziness, depression, inflammation of the iris, visual disturbances including changes in refraction (in some cases due to the suppression of miotic therapy), decreased heart rate, fainting, difficulty breathing (dyspnea), indigestion, kidney stones (often marked by a sudden onset of severe pain and cramps in the lower back and/or sides, groin or abdomen).
Rare(may affect up to 1 in 1,000 people)
Systemic lupus erythematosus (an immune disease that can cause inflammation of internal organs), tingling or numbness of the hands or feet, insomnia, nightmares, memory loss, increased signs and symptoms of myasthenia gravis (muscle disorder), decreased sexual desire, stroke, transient myopia that resolves when therapy is stopped, detachment of the layer under the retina that contains blood vessels after filtration surgery that can cause visual disturbances, drooping eyelid (making the eye stay half-closed), double vision, eyelid crusts, swelling of the cornea (with symptoms of visual disturbances), low eye pressure, ringing in the ears, low blood pressure, changes in heart rate or speed, congestive heart failure (heart disease with shortness of breath and swelling of feet and legs due to fluid accumulation), edema (fluid accumulation), cerebral ischemia (reduced blood flow to the brain), chest pain, strong and/or irregular heartbeats (palpitations), heart attack, Raynaud's phenomenon, swollen and/or cold hands and feet and decreased circulation in your arms and legs, leg cramps and/or leg pain when walking (claudication), difficulty breathing, respiratory failure, rhinitis, nosebleeds, constriction of airways in the lungs, cough, throat irritation, dry mouth, diarrhea, contact dermatitis, hair loss, skin rash with a silvery-white appearance (psoriasiform rash), Peyronie's disease (which can cause curvature of the penis), allergic reactions such as skin rash, hives, itching, in rare cases possible swelling of the lips, eyes, and mouth, wheezing, or severe skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis).
As with other eye medicines, timolol is absorbed into the bloodstream. This may cause side effects similar to those seen with oral beta-blockers. The incidence of side effects after topical ocular administration is lower than when, for example, medicines are administered orally or by injection. The following side effects were observed with beta-blocker medicines for the treatment of eye disorders:
Not known(frequency cannot be estimated from the available data)
Low blood sugar levels, hallucinations, heart failure, a type of heart rhythm disorder, increased heart rate, increased blood pressure, abdominal pain, vomiting, muscle pain not caused by exercise, sexual dysfunction.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Dorzolamida/Timolol Stulln
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the packaging and bottle after CAD. The expiration date is the last day of the month indicated.
Do not store at a temperature above 30°C
Bottle 5 ml: can be used up to 4 weeks after first opening.
Bottle 10 ml: can be used up to 8 weeks after first opening.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and unused medicines at the pharmacy's SIGRE point. In case of doubt, ask your pharmacist how to dispose of the packaging and unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition ofDorzolamida/Timolol Stulln
- The active ingredients are dorzolamida and timolol.
Each ml contains 20 mg of dorzolamida (as 22.26 mg of dorzolamida hydrochloride) and 5 mg of timolol (as 6.83 mg of timolol maleate).
- The other components are hydroxyethylcellulose (4000 - 5000 mPa·s), mannitol, sodium citrate, sodium hydroxide (for pH adjustment), and water for injectable preparations. Benzalkonium chloride is added as a preservative.
Appearance of the Product and Package Contents
Dorzolamida/Timolol Stulln is a clear, almost colorless, slightly viscous solution, practically free of visible particles.
Available package sizes: 1, 3, and 6 bottles of 5 ml eye drops in solution or 1, 2, and 3 bottles of 10 ml. Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Pharma Stulln GmbH
Werksstrasse 3
92551 Stulln
Germany
This medication is authorized in the member states of the European Economic Area under the following names:
Austria: Dorzolamid + Timolol Stulln 20 mg/ml + 5 mg/ml Augentropfen, Lösung
Germany: Dorzocomp-Stulln 20 mg/ml + 5 mg/ml Augentropfen, Lösung
France: Dorzolamide + Timolol Stulln 20 mg/ml + 5 mg/ml collyre en solution
Netherlands: Dorzolamide + Timolol Stulln 20 mg/ml + 5 mg/ml oogdruppels, oplossing
Poland: Dorzolamidum + Timololum Stulln
Spain: Dorzolamida/Timolol Stulln 20 mg/ml + 5 mg/ml eye drops in solution
Date of the Last Revision of this Leaflet:November 2023
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DORZOLAMIDE/TIMOLOL STULLN 20 mg/mL + 5 mg/mL EYE DROPS SOLUTIONDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for DORZOLAMIDE/TIMOLOL STULLN 20 mg/mL + 5 mg/mL EYE DROPS SOLUTION
Discuss questions about DORZOLAMIDE/TIMOLOL STULLN 20 mg/mL + 5 mg/mL EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions