
COMBIPRASAL 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION


How to use COMBIPRASAL 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for theuser
COMBIPRASAL 0.5 mg/2.5 mg
nebulizer inhalation solution
Read the entire leaflet carefully before startingto usethis medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again. If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is COMBIPRASAL and what is it used for
- What you need to know before starting to use COMBIPRASAL
- How to use COMBIPRASAL
- Possible side effects
- Storage of COMBIPRASAL
- Package contents and additional information
1. What is COMBIPRASAL and what is it used for
This medication comes in single-dose vials containing a clear, colorless solution for nebulizer inhalation. Combiprasal contains 0.5 mg of ipratropium bromide (as monohydrate) and 2.5 mg of salbutamol (as sulfate).
COMBIPRASAL belongs to a group of medications called bronchodilators that help open the airways in the lungs, making it easier for you to breathe.
It is indicated for the treatment of reversible bronchospasm associated with chronic obstructive pulmonary disease (COPD) in patients who require more than one bronchodilator.
2. BEFORE USING COMBIPRASAL
Do not use COMBIPRASAL
- If you are allergic (hypersensitive) to ipratropium bromide, salbutamol sulfate, atropine, or any other component of Combiprasal.
- If you have a heart condition called hypertrophic obstructive cardiomyopathy (when the heart does not work properly due to inflammation of the heart muscle).
- If you have a rapid and irregular heartbeat (known as tachyarrhythmia).
Warnings and precautions
Lactic acidosis has been observed with high therapeutic doses of salbutamol, mainly in patients treated for acute bronchospasm (see sections 3 and 4). The increase in lactate levels can lead to respiratory failure and hyperventilation.
Tell your doctor immediately if you feel that the medication is not working as usual and you need to use the nebulizer more often than your doctor has recommended.
Be careful with COMBIPRASAL
Tell your doctor before starting treatment with this medication:
- If you have a history of heart disease, irregular heartbeat, or angina pectoris.
- If you are pregnant, planning to become pregnant, or breastfeeding.
- Immediate allergic reactions, such as hives, angioedema, skin rash, cough, wheezing, and difficulty breathing (bronchospasm) and swelling of the mouth and throat (oropharyngeal edema), may occur.
- If you experience acute difficulty breathing that worsens rapidly, you should consult your doctor immediately.
- If you have diabetes.
- If you have an overactive thyroid gland.
- If you have a condition called pheochromocytoma, a tumor that produces chemicals that can cause fatigue, high blood pressure, and rapid heartbeat.
- If you have cystic fibrosis, as you may be more prone to gastrointestinal motility disorders.
- If you are prone to increased eye pressure (narrow-angle glaucoma). You should avoid getting the solution in your eyes. This can cause eye pain or discomfort, blurred vision, halos, or colored images, along with eye redness. If you experience any of these symptoms, you should stop treatment and consult your doctor immediately.
- If you have prostate enlargement (hyperplasia).
- If you have a disorder that makes it difficult to pass urine.
Using other medications
Tell your doctor or pharmacist if you are taking or have recently taken other medications, including those purchased without a prescription.
Some medications may interact with Combiprasal. It is essential to inform your doctor if you are taking any of the following medications:
- Xanthine derivatives, such as theophylline.
- Medications containing beta-blockers, such as propranolol or timolol, as they may reduce the effectiveness of this medication.
- Diuretics, such as furosemide or indapamide.
- Digoxin, used to treat heart failure.
- Other medications that help you breathe more easily, such as terbutaline.
- Medications containing anticholinergics (used to treat asthma, irritable bowel syndrome, Parkinson's disease, and incontinence).
- Certain medications for treating depression (known as monoamine oxidase inhibitors and tricyclic antidepressants).
- Anesthetic medications, such as halothane.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before taking any medication.
The use of Combiprasal is not recommended during pregnancy or breastfeeding. If necessary, your doctor will assess the convenience of using it.
Driving and using machines
No studies have been conducted on the effects on the ability to drive and use machines. However, do not drive or use machines until you know how the medication affects you.
Use in athletes
Athletes are informed that this medication contains salbutamol, which may result in a positive doping test.
3. How to use COMBIPRASAL
Follow your doctor's instructions for administering Combiprasal exactly. Consult your doctor or pharmacist if you have any questions.
Combiprasal should be used as needed and not on a regular basis.
If your asthma is active (for example, you have symptoms or frequent crises, such as difficulty breathing that makes it hard to speak, eat, or sleep, cough, wheezing, chest tightness, or limited physical capacity), you should inform your doctor immediately, who may start you on a medication or increase the treatment dose, such as an inhaled corticosteroid, to control your asthma.
Tell your doctor as soon as possible if your medication seems to be not working as well as usual (for example, if you need higher doses to relieve your respiratory problems or if your inhaler does not provide relief for at least 3 hours), as your asthma may be worsening, and you may need a different medication.
If you use Combiprasal more than twice a week to treat your asthma symptoms, excluding preventive use before exercise, this indicates poorly controlled asthma and may increase the risk of severe asthma attacks (asthma worsening) that can have serious complications and can be life-threatening. You should contact your doctor as soon as possible to review your asthma treatment.
If you use a daily medication to reduce inflammation in your lungs, such as an "inhaled corticosteroid," it is essential to continue using it regularly, even if you feel better.
Your doctor may instruct you to use your nebulizer regularly, either daily or only when you have difficulty breathing.
Adults, including the elderly, and children over 12 years:The recommended dose is 1 single-dose vial, 3 or 4 times a day. In severe cases, your doctor may increase the dose to 1 single-dose vial, 4 times a day.
There is no experience with the use of this medication in children under 12 years.
If you do not achieve significant improvement or your condition worsens, you should consult your doctor.
Instructions for correct administration of the preparation:
Your medication is for inhalation via a nebulizer or ventilator and should not be injected or swallowed.
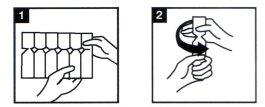
- Prepare your nebulizer according to the manufacturer's instructions and your doctor's instructions. Ensure the nebulizer device is clean.
- Take a plastic strip with vials from the box, open it, and remove one of the vials (Fig. 1). Leave the remaining vials on the strip and return it to the box.
- Take the vial and open it by twisting the top (Fig.2).
- Unless your doctor gives you other instructions, add all the liquid from the plastic vial to the nebulizer solution container.
- Use the nebulizer according to your doctor's instructions. Discard the empty plastic vial.
- After using the nebulizer, clean it according to the manufacturer's instructions.
If you think the effect of Combiprasal is too strong or too weak, tell your doctor or pharmacist.
If you use more COMBIPRASAL than you should:
If you use more than you should, you may experience changes in heart rhythm, palpitations, tremors, increased or decreased blood pressure, changes in pulse, angina pectoris, hot flashes, dry mouth, and visual accommodation disorders.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount administered. Contact your doctor or the nearest Hospital Emergency Service. Bring this leaflet or a vial of this medication with you, so the treating doctor knows what you are taking.
If you forget to use COMBIPRASAL
If you forget to use Combiprasal, use the next dose when it is due or sooner if you start to have difficulty breathing.
Do not take a double dose to make up for forgotten doses.
4. Possible side effects
Like all medications, Combiprasal can cause side effects, although not everyone will experience them.
The following side effects have been reported:
Common (affect 1 in 10 people)
- Nervousness
- Headache (cephalalgia)
- Cough
- Dry mouth
Uncommon (affect less than 1 in 100 people)
- Dizziness
- Tremors
- Hoarseness
- Changes in heart rhythm (tachycardia, palpitations, arrhythmia)
- Throat irritation
- Nausea
- Urinary retention
Rare (affect less than 1 in 1000 people)
- Allergic reactions (hypersensitivity)
- Decreased potassium levels in the blood (hypokalemia)
- Psychiatric disorders
- Increased intraocular pressure
- Narrow-angle glaucoma
- Eye pain
- Pupil dilation (mydriasis)
- Paradoxical bronchospasm
- Vomiting
- Gastrointestinal motility disorders
- Skin rash and increased sweating
- Muscle pain and weakness
- Muscle cramps
- Increased or decreased blood pressure
The following side effects may also occur, but their frequency is unknown:
- Some people may occasionally experience chest pain (due to heart problems such as angina pectoris). Tell your doctor if you develop these symptoms while being treated with COMBIPRASAL, but do not stop taking this medication unless your doctor tells you to.
- A condition known as lactic acidosis, which can cause stomach pain, hyperventilation, difficulty breathing, despite possible improvement in wheezing, cold hands and feet, irregular heartbeat, or thirst.
Reporting side effects:
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: http://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of COMBIPRASAL
Keep out of the reach and sight of children.
Do not store above 25°C.
Keep in the outer packaging to protect from light.
The vials should be opened immediately before use, and any remaining solution should be discarded.
Expiry date:
Do not use Combiprasal after the expiry date stated on the packaging after "Cad.:" The expiry date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and any unused medications in the pharmacy's SIGRE collection point. If you have any questions, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. ADDITIONAL INFORMATION
Combiprasal composition:
- The active ingredients are ipratropium bromide and salbutamol.
- The other ingredients (excipients) are: sodium chloride, hydrochloric acid, and water for injection.
Each 2.5 ml vial of Combiprasal contains 0.5 mg of ipratropium bromide (equivalent to 0.52 mg of ipratropium bromide monohydrate) and 2.5 mg of salbutamol (equivalent to 3 mg of salbutamol sulfate).
Appearance of the product and package contents:
Combiprasal is presented in strips of 10 vials containing a clear, colorless solution inside an aluminum pouch.
Each box contains 20 vials.
Marketing authorization holder:
Laboratorio ALDO-UNIÓN, S.L.
Baronessa de Maldà, 73
08950 Esplugues de Llobregat
Barcelona (Spain)
Manufacturer:
Laboratoire Unither
Espace Industriel Nord
151 rue André Durouchez-CS 28028
80084 AMIENS Cedex 2
France
This leaflet was approved in February 2018.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price13.88 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COMBIPRASAL 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Genetic S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Neutec Inhaler Ireland LimitedPrescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Cipla EuropePrescription required
Online doctors for COMBIPRASAL 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION
Discuss questions about COMBIPRASAL 0.5 mg/2.5 mg SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












