
COLIRCUSI PILOCARPINE 20 mg/ml EYE DROPS SOLUTION


How to use COLIRCUSI PILOCARPINE 20 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
COLIRCUSI PILOCARPINE 20 mg/ml eye drops solution
pilocarpine hydrochloride
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What COLIRCUSI PILOCARPINE is and what it is used for
- What you need to know before starting to use COLIRCUSI PILOCARPINE
- How to use COLIRCUSI PILOCARPINE
- Possible side effects
- Storage of COLIRCUSI PILOCARPINE
- Contents of the pack and additional information
1. What COLIRCUSI PILOCARPINE is and what it is used for
Colircusi Pilocarpine is an eye drop that contains an active substance (pilocarpine hydrochloride) belonging to a group of medications known as miotics, used to constrict the pupil and reduce the pressure inside the eye.
It is used as a miotic: to counteract pupillary dilation that occurs after surgical operation or eye examination.
In case of accidents or operations where prolapse (protrusion) of the iris must be avoided.
As a differential diagnosis of iris diseases, as it produces pupillary constriction.
It is also used as an ocular hypotensive: to reduce pressure before surgical operation and prevent pressure increase in the eye during laser surgery.
For the treatment of acute (angle-closure) or chronic (open-angle) glaucoma.
2. What you need to know before starting to use COLIRCUSI PILOCARPINE
Do not use Colircusi Pilocarpine
- If you are allergic to pilocarpine or any of the other components of this medication (listed in section 6).
- If you have inflammation of the iris or anterior uvea (one of the superficial layers of the eye).
- If you have pupillary block glaucoma (resistance of the flow of eye fluid through the pupil).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colircusi Pilocarpine.
- Use this medication only in your eye(s).
- If you have had a retinal detachment or are prone to it, or have other retinal diseases.
- If you are young and myopic (nearsighted).
(You will likely undergo a fundus examination before starting treatment with this medication).
- If you suffer any damage to the cornea or other parts of the eye.
- Consult your doctor if you have any of the following conditions, as they may worsen:
- Problems with the outflow of aqueous humor (fluid inside the eye)
- Heart problems
- Hypertension or hypotension
- Asthma or other pulmonary diseases
- Urination difficulties or inability
- Stomach ulcers (peptic ulcers)
- Hyperthyroidism
- Gastrointestinal spasm (painful digestive contraction)
- Parkinson's disease
- Recent myocardial infarction.
- If you drive at night or perform hazardous tasks with little light, as this medication may cause blurred vision and sensitivity to light.
- If you have dark eyes, you may need a higher concentration of this medication or apply it more frequently to produce the desired effect. Consult your doctor, as there may be a risk of overdose.
- If you use this medication for a long period, irritation or allergic reactions may occur. In these cases, you should discontinue treatment.
Children
Do not use in children, as safety and efficacy have not been established in this population.
Other medications and Colircusi Pilocarpine
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Especially, inform your doctor if you are using:
- Ophthalmic non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, as they may decrease the effect of this eye drop.
- Beta-adrenergic antagonist drugs (used to treat hypertension and heart diseases), as they may increase the risk of heart problems.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not use this medication during pregnancy or breastfeeding, unless your doctor considers it necessary.
Driving and using machines
The influence of this medication on the ability to drive and use machines is significant.
After application, this medication may cause blurred vision and difficulty adapting to darkness. If these effects occur, do not drive or use machines until they have disappeared.
In particular, you should be cautious when driving at night or performing hazardous tasks with little lighting.
COLIRCUSI PILOCARPINE contains benzalkonium chloride
This medication contains 0.1 mg of benzalkonium chloride per ml.
Benzalkonium chloride may be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medication and wait 15 minutes before reinserting them.
Benzalkonium chloride may cause eye irritation, especially if you have dry eye or other corneal diseases (transparent layer of the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medication.
3. How to use COLIRCUSI PILOCARPINE
Follow the administration instructions of this medication indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The following measure is useful to limit the amount of medication that passes into the bloodstream after applying the eye drop:
Keep your eyes closed while gently pressing the lacrimal canal with your finger for at least 2 minutes.
The recommended dose is:
Adults, including elderly patients
- Induction of miosis: 1 or 2 drops, spaced 5 minutes apart, in the affected eye(s).
- Prevention of ocular hypertension in case of laser surgery: 1 or 2 drops, spaced 5 minutes apart, in the affected eye(s), 15 to 60 minutes before surgery.
- Acute glaucoma: 1 drop in the affected eye(s), up to 3 times in a 30-minute period (you may need prior treatment).
- Ocular hypertension and elevated intraocular pressure due to open-angle glaucoma: 1 drop in the affected eye(s), up to 4 times a day.
Your doctor may modify the number of daily applications and indicate the duration of treatment with this eye drop. Do not discontinue treatment before, unless your doctor indicates so.
Recommendations for use
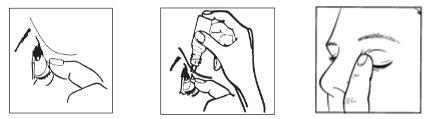
1 2 3
- Take the bottle (dropper container).
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back. Gently separate the eyelid from the eye with your finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or surrounding areas, with the dropper. The drops may become contaminated.
- Gently squeeze the base of the bottle with your index finger to release one drop at a time (figure 2).
- After using this eye drop, close your eye and gently press the edge of the eye near the nose with your finger for 2 minutes. This helps prevent the eye drop from passing into the rest of the body (figure 3).
- If you are applying drops in both eyes, repeat all the previous steps with the other eye.
- Close the bottle immediately after use.
If a drop falls outside the eye, try again.
Use in case of hepatic and/or renal insufficiency
Safety and efficacy have not been established in patients with severe hepatic or renal insufficiency.
If you are using other ophthalmic medications, wait at least 5 minutes between the administration of this medication and other ophthalmic medications. Ophthalmic ointments should be administered last.
If you use more COLIRCUSI PILOCARPINE than you should, you can eliminate it by washing your eyes with warm water. Do not apply more drops until it is time for your next dose.
Symptoms of overdose or accidental ingestion may include: headache, salivation, sweating, fainting, slow heart rate, hypotension, abdominal cramps, vomiting, asthma, and diarrhea.
Treatment of overdose is supportive. In case of severe systemic toxicity, treatment with anticholinergics may be necessary.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone 91 562 04 20, indicating the medication and the amount used.
If you forget to use COLIRCUSI PILOCARPINE
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next dose as scheduled. However, if it is almost time for the next dose, do not apply the missed dose and continue with your regular schedule.
If you have any further questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone may experience them.
The following side effects have been observed with this medication:
Very common (may affect more than 1 in 10 people):
- Eye effects: blurred vision.
- General effects: headache.
Common (may affect up to 1 in 10 people):
- Eye effects: floating bodies in the fluid inside the eye (appearance of spots in vision), reduced vision (especially in elderly patients or those with opacities), eye pain, abnormal vision of lights, eye irritation, and eye redness.
- General effects: dizziness and nausea.
Uncommon (may affect up to 1 in 100 people):
- Eye effects: retinal detachment, bleeding inside the eye, fluid detachment from the interior of the eye, eyelid swelling, decreased pupil size, dazzle, and abnormal sensation in the eye.
Frequency not known (cannot be estimated from available data):
- Eye effects: increased pressure in the eye and corneal swelling.
- General effects: vomiting.
Other side effects that may occur with this type of medication
Prolonged administration of miotics may lead to an increased risk of cataracts, iris cysts, or retinal detachment in patients with pre-existing retinal disease.
A paradoxical increase in pressure in the eye may occur in patients with altered outflow.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of COLIRCUSI PILOCARPINE
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date stated on the bottle and carton after "EXP". The expiration date is the last day of the month indicated.
Do not store above 25°C.
To avoid infections, discard the bottle 4 weeks after opening it for the first time.
Write the opening date of the bottle in the space provided on the carton.
Medications should not be disposed of through wastewater or household waste. Place the packaging and any unused medication in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Contents of the pack and additional information
Composition of COLIRCUSI PILOCARPINE
- The active ingredient is pilocarpine hydrochloride. One ml of solution contains 20 mg of pilocarpine hydrochloride (2%).
- The other ingredients are benzalkonium chloride, povidone, sodium chloride, borax, and purified water.
Appearance of the product and contents of the pack
Colircusi Pilocarpine is an eye drop solution (clear and colorless or slightly yellowish liquid).
It is presented in a dropper container (plastic bottle with a cap) in a carton.
Each container contains 10 ml of eye drop solution.
MARKETING AUTHORIZATION HOLDER:
Mizar Farmacéutica, S.L.
Gran Via de les Corts Catalanes, 764
08013 – Barcelona, Spain
MANUFACTURER
Siegfried El Masnou, S.A.
C/ Camil Fabra, 58
08320 El Masnou - Barcelona, Spain
or
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona, Spain
or
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg, Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 – Barcelona, Spain
Date of the last revision of this package leaflet:February 2022.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price2.5 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIRCUSI PILOCARPINE 20 mg/ml EYE DROPS SOLUTIONDosage form: INJECTABLE, 20 mg acetylcholine chlorideActive substance: acetylcholineManufacturer: Fidia Farmaceutici S.P.A.Prescription requiredDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYE DROP, 2 mg/mlActive substance: brimonidineManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for COLIRCUSI PILOCARPINE 20 mg/ml EYE DROPS SOLUTION
Discuss questions about COLIRCUSI PILOCARPINE 20 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















