
CLINDAMYCIN KALCEKS 150 mg/mL Injectable Solution and Perfusion Solution

How to use CLINDAMYCIN KALCEKS 150 mg/mL Injectable Solution and Perfusion Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Clindamicina Kalceks 150 mg/ml solution for injection and infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Clindamicina Kalceks and what is it used for
- What you need to know before you use Clindamicina Kalceks
- How to use Clindamicina Kalceks
- Possible side effects
- Storage of Clindamicina Kalceks
- Contents of the pack and other information
1. What is Clindamicina Kalceks and what is it used for
This medicine contains the active substance clindamycin (as clindamycin phosphate), which is an antibiotic. It is used to treat infections.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as flu or common cold.
It is important that you follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw medicines down the drain or in the trash.
Clindamicina Kalceks is used to treat the following severe infections in adults and children from 1 month of age:
- bone and joint infections
- chronic sinusitis caused by anaerobic microorganisms
- lower respiratory tract infections
- complicated abdominal infections
- pelvic and female genital tract infections
- complicated skin and soft tissue infections.
Clindamicina Kalceks may be used for prophylaxis in surgery in case of allergy to beta-lactams.
2. What you need to know before you use Clindamicina Kalceks
You should not be given Clindamicina Kalceks
- if you are allergic to clindamycin, lincomycin (another antibiotic), or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or nurse before starting treatment with clindamycin if:
- you have liver or kidney problems;
- you have muscle problems (e.g., you have a muscle weakness called myasthenia gravis or Parkinson's disease);
- you have had gastrointestinal diseases (e.g., colon inflammation) in the past;
- you have any type of allergy, e.g., hypersensitivity to penicillin, because in individual cases, allergic reactions to clindamycin have been reported in people with known hypersensitivity to penicillin;
- you have asthma, eczema, or hay fever.
Talk to your doctor if you are not sure if you are affected by any of the above warnings.
Tell your doctor or nurse immediately if you experience:
- signs of a severe allergic reaction such as wheezing, difficulty breathing, swelling of eyelids, face, or lips, skin rash, or itching (see section 4). These can occur even after the first administration. In this case, your doctor will immediately stop treatment with clindamycin and initiate standard emergency measures;
- severe skin rash with irregular red patches or blisters containing pus and extensive skin peeling, fever, cough, feeling unwell, and swelling of the gums, tongue, or lips (see section 4);
- diarrhea during or up to 3 weeks after treatment, especially if it is accompanied by mucus or blood in the stool. This can be a sign of a severe colon infection (colitis). It is more likely to occur in immunocompromised patients and/or the elderly (over 60 years of age).
Acute kidney problems may occur. Tell your doctor about any medication you are currently taking and if you have any kidney problems. If you experience decreased urine production, fluid retention that causes swelling of the legs, ankles, or feet, shortness of breath, or nausea, you should contact your doctor immediately.
Prolonged treatment and repeated use of clindamycin may cause a skin and mucous membrane infection with pathogens that are not sensitive to clindamycin. It may also cause the development of a fungal infection.
This medicine is not suitable for the treatment of brain fever (meningitis).
During long-term treatment, your doctor will periodically check your liver and kidney function.
To avoid undesirable effects, clindamycin will be administered by slow intravenous infusion.
Children
Safety and dosage have not been established in infants under 1 month of age.
Other medicines and Clindamicina Kalceks
Tell your doctor or nurse if you are using, have recently used, or might use any other medicines.
Especially if you are using any of the following medicines:
- muscle relaxants (used during operations to help relax muscles). Concomitant use with clindamycin may result in unexpected and potentially fatal incidents during surgery. Therefore, if you are in the hospital for an operation or a hospital procedure, tell your doctor that you are receiving clindamycin.
- warfarin or similar medicines: used to thin the blood. You may be more likely to bleed. Your doctor may want to perform periodic blood tests to check your blood's clotting ability;
- erythromycin (an antibiotic);
- rifampicin (to treat tuberculosis);
- cyclosporin/tacrolimus (used after organ transplants).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before you are given this medicine.
Your doctor will only prescribe clindamycin during pregnancy if it is absolutely necessary. This medicine may have a negative effect on the intestinal flora of the breastfed infant. Your doctor will carefully weigh the benefits of breastfeeding for the infant and the benefits and risks expected from treatment with clindamycin for the mother.
Driving and using machines
The influence of this medicine on the ability to drive and use machines is negligible or nonexistent.
Clindamicina Kalceks contains sodium
This medicine contains 6.5 mg of sodium (a major component of table/cooking salt) per ml of solution. This is equivalent to 0.33% of the maximum recommended daily sodium intake for an adult.
3. How to use Clindamicina Kalceks
Your doctor will decide the correct treatment dose of clindamycin for you.
This medicine will be administered by a doctor or nurse, either by injection into the muscle or by slow intravenous infusion (drip). The medicine will be diluted before infusion into a vein. The infusion will last from 10 to 60 minutes.
Adults
- for the treatment of less complicated infections:
the usual dose is 1,200-1,800 mg per day administered in 3 or 4 equal doses
- for the treatment of severe infections:
the usual dose is 2,400-2,700 mg per day administered in 2, 3, or 4 equal doses.
In potentially life-threatening infections, the dose administered intravenously may be increased up to 4,800 mg per day.
The dose for the prophylaxis of postoperative infections will be determined by your doctor, depending on the type and duration of the surgical procedure.
By intramuscular route, the recommended maximum single dose is 600 mg.
By intravenous route, the recommended maximum dose for a one-hour infusion is 1,200 mg.
Use in elderly patients
No dose adjustment is necessary in elderly patients with normal liver and kidney function.
Patients with hepatic and/or renal impairment
Generally, no dose adjustment is necessary in case of mild or moderate hepatic or renal impairment.
Your doctor will monitor kidney function in patients with severe renal impairment.
In patients with severe hepatic impairment, your doctor will monitor liver function and, when possible, the levels of the medicine in the blood. If necessary, your doctor will adjust the dose or dosing intervals.
Use in children and adolescents
Children over 1 month to 12 years
20-40 mg/kg body weight per day administered in 3 or 4 equal doses.
The dose of clindamycin in children should be based on the total body weight, regardless of obesity. In severe infections, it is recommended to administer at least 300 mg/day in children, regardless of body weight. The total daily dose should not exceed the maximum recommended daily dose for adults.
Adolescents over 12 years
The doses in adolescents over 12 years should be the same as in adults, taking into account possible dose adjustments based on liver function. In adolescent patients with low weight, between 12 and 18 years, it is not recommended to exceed the maximum dose of 40 mg/kg/day. The total daily dose should not exceed the maximum recommended daily dose for adults.
If you are given too much Clindamicina Kalceks
This medicine will always be administered under carefully controlled conditions. However, if you think you have been given too much medicine, tell your doctor or nurse immediately.
If you miss a dose of Clindamicina Kalceks
This medicine will be administered by a doctor or nurse. However, if you think you have missed a dose, tell your doctor or nurse.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Tell your doctor or nurse immediatelyif you experience any of the following side effects, as you may need urgent medical attention:
Common(may affect up to 1 in 10 people)
- Gastrointestinal disorders
Severe, persistent, or bloody diarrhea (which may be associated with stomach pain or fever). This can occur during or after treatment with antibiotics and can be a sign of severe intestinal inflammation (pseudomembranous colitis).
Uncommon(may affect up to 1 in 100 people)
- Cardiovascular disorders
Low blood pressure (feeling dizzy, dizzy, or faint) or sudden pain or pressure in the chest, difficulty breathing, dizziness, fainting, nausea, or vomiting (signs of cardiac arrest). This can occur if the medicine is administered too quickly.
Frequency not known(cannot be estimated from the available data)
- Allergic reactions
Signs of a severe allergic reaction, such as sudden wheezing, difficulty breathing, swelling of eyelids, face, or lips, skin rash, or itching (which can affect the whole body)
- Skin disorders
Signs of severe and potentially life-threatening skin reactions, such as severe skin rash with irregular red patches or blisters containing pus and extensive skin peeling, fever, cough, feeling unwell, and swelling of the gums, tongue, or lips
- Hepatic disorders
Yellowing of the skin and the whites of the eyes (jaundice)
- Renal disorders
Fluid retention that causes swelling of the legs, ankles, or feet, difficulty breathing, or nausea.
- Changes in blood counts
Increased risk of infections, which can manifest as fever, chills, sore throat, or mouth ulcers (can indicate that you have a low number of white blood cells in your body)
Other side effects
Common(may affect up to 1 in 10 people)
- Infection or inflammation of the veins in the leg (thrombophlebitis)
- Increased number of certain white blood cells (eosinophilia)
- Inflammation of the lining of the mouth
- Diarrhea
- Raised red skin rash
- Hardening at the injection site (after injection into the muscle)
- Changes in liver function (observed in a blood test)
Uncommon(may affect up to 1 in 100 people)
- Low number of white blood cells in the body (granulocytopenia)
- Impaired sense of taste
- Muscle relaxant effect
- Abdominal pain
- Inflammation of the esophagus lining
- Nausea
- Vomiting
- Hives
- Inflammation and redness of the skin (erythema multiforme)
- Itching
- Pain at the injection site
- Abscess at the injection site (after injection into the muscle)
Rare(may affect up to 1 in 1,000 people)
- Swelling, especially of the face and throat, wheezing, and/or difficulty breathing (angioedema)
Very rare(may affect up to 1 in 10,000 people)
- Indigestion
Frequency not known(cannot be estimated from the available data)
- Intestinal infection (colon) caused by a bacterium.
- Vaginal infection
- Unexplained bruising or bleeding for a longer time than usual or small red-purple spots on the skin (can indicate that you have a low number of platelets in the blood)
- Allergic reactions
- Changes in smell
- Flat or slightly raised red-pink skin rash
- Irritation at the injection site
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Clindamicina Kalceks
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the ampoule and on the carton after EXP. The expiry date is the last day of the month shown.
Do not store above 25 °C. Do not refrigerate or freeze.
Store the ampoules in the outer packaging to protect them from light.
Shelf life after opening the ampoule: the product should be used immediately.
Shelf life after dilution
The chemical and physical stability in use has been demonstrated for 48 hours at 25 °C and 2-8 °C.
From a microbiological point of view, the product should be used immediately. If not used immediately, the storage times and conditions before use are the responsibility of the user and normally should not exceed 24 hours at 2 to 8 °C, unless the dilution has been made under controlled and validated aseptic conditions.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Clindamicina Kalceks
- The active ingredient is clindamycin.
Each ml of solution contains 150 mg of clindamycin (as clindamycin phosphate).
Each 2 ml ampoule contains 300 mg of clindamycin (as clindamycin phosphate).
Each 4 ml ampoule contains 600 mg of clindamycin (as clindamycin phosphate).
- The other components are disodium edetate, sodium hydroxide (for pH adjustment), and water for injectable preparations.
Appearance of the Product and Container Contents
Transparent solution, colorless to almost colorless, practically free from visible particles.
2 ml or 4 ml of solution packaged in colorless glass ampoules with a breaking point.
Each container contains 1, 5, or 10 (clinical container) ampoules.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
AS KALCEKS
Krustpils iela 71E,
Riga, LV‑1057,
Latvia
Tel.: +371 67083320
E-mail: [email protected]
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Grindeks Kalceks España, S.L.
c/ José Abascal, 58 2º dcha
28003 Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Denmark Clindamycin Kalceks
Austria, Germany Clindamycin Kalceks 150 mg/ml Injektions-/Infusionslösung
Croatia Klindamicin Kalceks 150 mg/ml otopina za injekciju/infuziju
Finland, Norway Clindamycin Kalceks
France CLINDAMYCINE KALCEKS 600 mg/4 mL, solution injectable/pour perfusion
Hungary Clindamycin Kalceks 150 mg/ml oldatos injekció vagy infúzió
Ireland Clindamycin 150 mg/ml solution for injection/infusion
Italy Clindamicina Kalceks
Latvia Clindamycin Kalceks 150 mg/ml šķidums injekcijām/infūzijām
Netherlands Clindamycine Kalceks 150 mg/ml oplossing voor injectie/infusie
Slovenia Klindamicin Kalceks 150 mg/ml raztopina za injiciranje/infundiranje
Spain Clindamicina Kalceks 150 mg/ml solución inyectable y para perfusión EFG
Date of the last revision of this leaflet: June 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Consult the Summary of Product Characteristics for complete information on prescription.
Method of Administration
Intramuscular (IM) injection or intravenous (IV) infusion.
For intramuscular administration, clindamycin should be used undiluted. Administration of more than 600 mg in a single dose is not recommended.
Intramuscular administration is indicated when intravenous infusion is not possible for some reason.
For intravenous administration, Clindamicina Kalceks must be diluted before IV administrationand must be infused over at least 10-60 minutes. The concentration should not exceed 18 mg of clindamycin per ml of solution, and the infusion rate should not exceed 30 mg/min. It should never be administered as an intravenous bolus injection(may cause serious adverse effects). Intravenous infusions of more than 1,200 mg in one hour are not recommended.
Common Infusions
Dose | Diluent | Clindamycin Concentration | Minimum Infusion Time |
300 mg | 50 ml | 6 mg/ml | 10 minutes |
600 mg | 50 ml | 12 mg/ml | 20 minutes |
900 mg | 50-100 ml | 9 mg/ml to 18 mg/ml | 30 minutes |
1,200 mg | 100 ml | 12 mg/ml | 40 minutes |
For compatible diluents, see "Instructions for Use, Handling, and Disposal".
Incompatibilities
This medicinal product should not be mixed with other medicinal products, except those mentioned below in the "Instructions for Use, Handling, and Disposal" section.
The following medicinal products are physically incompatible with clindamycin phosphate: ampicillin, sodium phenytoin, barbiturates, aminophylline, calcium gluconate, magnesium sulfate, sodium ceftriaxone, ciprofloxacin, idarubicin hydrochloride, and ranitidine hydrochloride.
Instructions for Use, Handling, and Disposal
For single use. Discard any unused medicinal product.
The medicinal product should be visually inspected before use. Do not use if there are visible signs of deterioration (e.g., particles). Only clear solutions without visible particles should be used.
It can be diluted with:
- 9 mg/ml (0.9%) sodium chloride solution for infusion
- 50 mg/ml (5%) glucose solution for infusion
Instructions for opening the ampoule
- Turn the ampoule with the colored tip upwards. If solution remains in the top part of the ampoule, gently tap with your finger to make the solution pass to the lower part of the ampoule.
- Open the ampoule with both hands; while holding the lower part of the ampoule with one hand, use the other hand to break the upper part of the ampoule in the direction opposite to the colored point (see the following images).
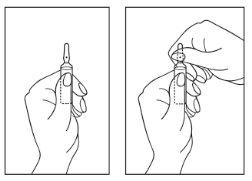
Disposal of unused medicinal product and all materials that have come into contact with it should be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLINDAMYCIN KALCEKS 150 mg/mL Injectable Solution and Perfusion SolutionDosage form: INJECTABLE, -Active substance: clindamycinManufacturer: Laboratorios Normon S.A.Prescription requiredDosage form: INJECTABLE, 600 mgActive substance: clindamycinManufacturer: Laboratorios Normon S.A.Prescription requiredDosage form: CAPSULE, 150 mgActive substance: clindamycinManufacturer: Neuraxpharm Spain S.L.Prescription required
Online doctors for CLINDAMYCIN KALCEKS 150 mg/mL Injectable Solution and Perfusion Solution
Discuss questions about CLINDAMYCIN KALCEKS 150 mg/mL Injectable Solution and Perfusion Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















