
CEFTAZIDIME QILU 2 g POWDER FOR INJECTION AND INFUSION SOLUTION

How to use CEFTAZIDIME QILU 2 g POWDER FOR INJECTION AND INFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ceftazidime Qilu 2 g powder for solution for injection and infusion EFG
ceftazidime
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ceftazidime Qilu and what is it used for
- What you need to know before you use Ceftazidime Qilu
- How to use Ceftazidime Qilu
- Possible side effects
- Storage of Ceftazidime Qilu
- Contents of the pack and further information
1. What is Ceftazidime Qilu and what is it used for
Ceftazidime is an antibiotic that is used in adults and children (including newborns) and works by killing the bacteria that cause infections. It belongs to a group of medicines called cephalosporins.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as flu or the common cold.
It is important that you follow the instructions regarding the dose, administration interval, and duration of treatment indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after finishing treatment, return it to the pharmacy for proper disposal. Do not throw medicines down the drain or in the trash.
Ceftazidime is used to treat serious bacterial infections of:
- the lungs or chest
- the lungs and bronchi in patients with cystic fibrosis
- the brain (meningitis)
- the ears
- the urinary tract
- the skin and soft tissues
- the abdomen and abdominal wall (peritonitis)
- the bones and joints
Ceftazidime can also be used:
- to prevent infections during prostate surgery in men.
- to treat patients with low white blood cell counts (neutropenia) and fever due to a bacterial infection.
2. What you need to know before you use Ceftazidime Qilu
Do not use Ceftazidime Qilu:
- if you are allergic to ceftazidime or any of the other ingredients of this medicine (listed in section 6).
- if you have had a severe allergic reaction to another antibiotic (penicillins, monobactams, and carbapenems), as you may also be allergic to ceftazidime.
→If you think you may be in one of these situations, tell your doctorbefore you are given Ceftazidime Qilu. If so, you should not receive this medicine.
Warnings and precautions
Talk to your doctor or nurse before you are given Ceftazidime Qilu.
While you are receiving this medicine, you should be aware of certain symptoms, such as allergic reactions, nervous system disorders, and gastrointestinal disorders, such as diarrhea. This will reduce the risk of possible problems. See the section Diseases that require special attentionin section 4. If you have had an allergic reaction to other antibiotics, you may also be allergic to ceftazidime.
Severe skin reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in relation to ceftazidime treatment. Seek medical attention immediately if you notice any of the symptoms related to these severe skin reactions described in section 4.If you need a blood or urine test
Ceftazidime may affect the results of glucose (sugar) tests in urine and a blood test called the Coombs test. If you are going to have tests:
→Tell the person taking the samplethat you have been given Ceftazidime Qilu.
Using Ceftazidime Qilu with other medicines
Tell your doctor if you are taking, have recently taken, or might take any other medicines,including those you can buy without a prescription.
You should not be given ceftazidime without first talking to your doctor if you are also taking:
- An antibiotic called chloramphenicol.
- A type of antibiotic called aminoglycoside(e.g., gentamicin or tobramycin).
- Diuretics called furosemide
→If you are in any of these situations, tell your doctor.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Your doctor will assess the benefit of treating you with ceftazidime against the risk to your child.
Driving and using machines
This medicine may cause side effects that affect your ability to drive or use machines, such as dizziness. Do not drive or use machines unless you are sure it won't affect you.
Ceftazidime Qilu contains sodium.
You should be aware of this if you are on a low-sodium diet.
Ceftazidime Qilu (concentration) | Amount per vial |
Ceftazidime Qilu 2 g | This medicine contains 104 mg of sodium (the main component of table salt and kitchen salt) in each vial, which is equivalent to 5.2% of the maximum recommended daily intake of sodium in food for an adult. |
3. How to use Ceftazidime Qilu
Ceftazidime Qilu is usually given to you by a doctor or nurse.
Ceftazidime Qilu 2 g can be given by injection into a vein or infusion.
Your doctor, nurse, or pharmacist will prepare Ceftazidime Qilu with water for injections or a suitable infusion liquid.
The recommended dose is:
The correct dose of ceftazidime will be decided by your doctor and depends on the severity and type of infection, whether you are taking other antibiotics, your weight and age, and how well your kidneys are working.
Newborns (0-2 months)
For every 1 kg of body weight, 25-60 mg of ceftazidime will be given per day, divided into two doses.
Infants (over 2 months) and childrenweighing less than 40 kg
For every 1 kg of body weight, 100-150 mg of ceftazidime will be given per day, divided into three doses. The maximum dose is 6 g per day.
Adults and adolescentsweighing 40 kg or more
1 g to 2 g of ceftazidime three times a day. The maximum dose is 9 g per day.
Patients over 65 years of age
The daily dose should not exceed 3 g per day, especially if you are over 80 years old.
Patients with kidney problems
You may be given a different dose than usual. Your doctor or nurse will decide the amount of Ceftazidime Qilu you need, based on the severity of your kidney disease. Your doctor will monitor you closely and may perform more frequent tests of kidney function.
If you use more Ceftazidime Qilu than you should
If you think you have been given too much medicine, contact your doctor or the nearest hospital immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken.
If you miss a dose of Ceftazidime Qilu
If you miss an injection of this medicine, you should receive it as soon as possible. You should not be given a double dose (two injections at the same time) to make up for the missed dose.
If you stop using Ceftazidime Qilu
Do not stop using Ceftazidime Qilu unless your doctor tells you to.
If you have any further questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Diseases that require special attention
The following serious side effects have been reported in a small number of people (the exact frequency is unknown):
- Severe allergic reaction. The signs include itchy, lumpy rash, swelling, sometimes on the face or mouth, causing difficulty breathing.
- Skin rashthat can form blisteringand appears as small targets(a dark center, surrounded by a lighter area, with a dark ring around the edge).
- A widespread rashaccompanied by blisteringand peeling of the skin, ulcers in the mouth, throat, nose, genitals, or eyes. These severe skin rashes can be preceded by fever and flu-like symptoms (These signs may correspond to Stevens-Johnson syndromeor toxic epidermal necrolysis).
- Nervous system disorders: tremors, seizures, and, in some cases, coma. These side effects have occurred in patients who were given too high a dose, especially in patients with kidney disease.
- Rare cases of severe hypersensitivity reactions have been reported, accompanied by severe rash, which may be associated with fever, fatigue, swelling of the face or lymph nodes, increased eosinophils (a type of white blood cell), effects on the liver, kidneys, or lungs (a reaction called DRESS).
- A widespread, red, itchy rashwith peeling, bumps under the skin, and blistering, accompanied by fever. The symptoms usually appear at the start of treatment (acute generalized exanthematous pustulosis).
-
Other side effects:
Frequent: may affect up to 1 in 10 people
- diarrhea
- swelling and redness around a vein
- red, itchy, lumpy rash
- pain, burning, swelling, or inflammation at the injection site
- If you are concerned about any of these symptoms, tell your doctor.
The frequent side effects that may appear in blood tests are:
- an increase in the count of a type of white blood cell (eosinophilia)
- an increase in the count of cells involved in blood clotting
- an increase in liver enzymes
Uncommon
- inflammation of the intestine, which can cause pain or diarrhea that may contain blood
- candidiasis (fungal infections in the mouth or vagina)
- headache
- dizziness
- stomach pain
- feeling unwell or dizzy
- fever and chills.
- If you experience any of these symptoms, tell your doctor.
The uncommon side effects that may appear in blood tests are:
- a decrease in the count of white blood cells
- a decrease in the count of platelets (the cells that help with blood clotting)
- an increase in the concentration of urea, ureic nitrogen, or serum creatinine in the blood
Rare
- inflammation or failure of the kidneys
Frequency not known: cannot be estimated from the available data.
- inflammation or failure of the kidneys
- tingling
- bad taste in the mouth
- yellowing of the whites of the eyes or skin
The side effects of unknown frequency that may appear in blood tests are:
- rapid destruction of red blood cells
- an increase in the count of a certain type of white blood cell
- a sudden decrease in the count of white blood cells
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use www.notificaram.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ceftazidime Qilu
Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the vial and carton after EXP. The expiry date is the last day of the month shown.
- Store below 25 °C.
- Keep the vials in the outer packaging to protect them from light.
- Reconstituted and diluted solution: your doctor, pharmacist, or nurse will prepare your medicine with water for injections or a compatible infusion liquid.
- The physical and chemical stability during use is 24 hours at a temperature between 2 °C and 8 °C and 2 hours at 25 °C.
- From a microbiological point of view, the product should be used immediately, unless the method of opening, reconstitution, and dilution prevents the risk of microbiological contamination. If not used immediately, the time and conditions of storage during use are the responsibility of the user.
- Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy. If you are unsure, ask your pharmacist how to dispose of containers and unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Ceftazidime Qilu
Ceftazidime Qilu is available in the following presentations: 2 g, 1 g, and 500 mg.
- The active substance is 2 g, 1 g, or 500 mg of ceftazidime (in the form of ceftazidime pentahydrate).
- The other ingredients are: sodium carbonate.
See section 2 for important information about sodium, one of the components of Ceftazidime Qilu.
Appearance and packaging
Ceftazidime Qilu 2 g powder for solution for injection and infusion is a sterile, crystalline powder, white or off-white in color, contained in a 50 ml capacity glass vial, with a butyl rubber stopper and an aluminum seal.
Available in packs of 1, 10, or 50 vials.
Not all pack sizes may be marketed.
Your doctor, pharmacist, or nurse will prepare the injection or infusion with water for injections or a suitable infusion liquid. Ceftazidime Qilu changes color when reconstituted, from light yellow to amber. This is completely normal.
Marketing authorization holder and manufacturer
QILU PHARMA SPAIN S.L.,
Paseo de la Castellana 40
8th floor, 28046 - Madrid
Spain
Local Representative
Sun Pharma Laboratories, S.L.
Rambla de Catalunya 53-55
08007 – Barcelona, Spain
Manufacturer
KYMOS, S.L
Ronda de Can Fatjó,
7B (Parque Tecnológico del Vallès),
Cerdanyola del Vallès, 08290,
Barcelona, Spain
NETPHARMALAB CONSULTING SERVICES
Carretera de Fuencarral 22
Alcobendas, 28108 - Madrid,
Spain
MIAS Pharma Limited
Suite 2, Stafford House, Strand Road, Portmarnock, Co. Dublin,
Ireland
Tillomed Malta Ltd.
Malta Life Sciences Park, LS2.01.06 Industrial Estate, San Gwann, SGN 3000,
Malta
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
UK | Ceftazidime 2 g powder for solution for injection/infusion |
DE | Ceftazidim Qilu 2 g powder for solution for injection/infusion |
ES | Ceftazidima Qilu 2 g powder for injectable solution and perfusion EFG |
FR | CEFTAZIDIME QILU 2 g, powder for injectable solution/for perfusion (IV) |
IT | Ceftazidima Qilu |
SE | Ceftazidim Qilu 2 g powder for injection/infusion solution, solution |
Date of last revision of this prospectus: 10/2024.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Ceftazidima Qilu 2 g powder for injectable solution and perfusion EFG
Ceftazidime
Please consult the detailed information of the Technical Sheet or Summary of Product Characteristics.
After reconstitution:
The physical and chemical stability during use is 24 hours at a temperature between 2 °C and 8 °C and 2 hours at 25 °C in water for injectable preparations or compatible liquids listed below.
From a microbiological point of view, the medicinal product should be used immediately, unless the method of opening, reconstitution, and dilution avoids the risk of microbiological contamination.
If not used immediately, the time and conditions of storage during use are the responsibility of the user.
After dilution:
The physical and chemical stability during use is 24 hours at a temperature between 2 °C and 8 °C and 2 hours at 25 °C in water for injectable preparations or compatible liquids listed below.
From a microbiological point of view, the medicinal product should be used immediately, unless the method of opening, reconstitution, and dilution avoids the risk of microbiological contamination.
If not used immediately, the time and conditions of storage during use are the responsibility of the user.
Special precautions for storage
Keep below 25 °C.
Keep the vials in the outer packaging to protect them from light.
Special precautions for disposal and other handling
As the product dissolves, carbon dioxide is released and a positive pressure develops. The small carbon dioxide bubbles in the reconstituted solution should be ignored.
Instructions for reconstitution
Consult the volumes for addition and concentrations of the solution in Tables 1 and 2, which may be useful when fractional doses are needed.
Table 1. Powder for injectable solution
Presentation | Amount of diluent to be added (ml) | Approximate concentration (mg/ml) | |
2 g | |||
Intravenous bolus | 10 ml | 170 |
Note:
- The resulting volume of the ceftazidime solution in the reconstituted medium increases as a consequence of the drug's displacement factor in the concentrations listed in mg/ml presented in the table above.
Table 2. Powder for perfusion solution
Presentation | Amount of diluent to be added (ml) | Approximate concentration (mg/ml) | |
2 g | |||
Intravenous infusion | 50 ml* | 40 |
- The addition should be performed in two stages.
Note:
- The resulting volume of the ceftazidime solution in the reconstituted medium increases as a consequence of the drug's displacement factor in the concentrations listed in mg/ml presented in the table above.
The color of the solutions varies from pale yellow to amber, depending on the concentration, diluents, and storage conditions used. The potency of the product is not negatively affected by these color variations, within the recommended indications.
Ceftazidime at concentrations between 1 mg/ml and 40 mg/ml is compatible with:
- Sodium chloride 0.9% (9 mg/ml) injectable solution
- Sodium lactate M/6 injectable solution
- Compound sodium lactate injectable solution (Hartmann's solution)
- 5% (50 mg/ml) glucose injectable solution
- 0.225% (2.25 mg/ml) sodium chloride and 5% (50 mg/ml) glucose injectable solution
- 0.45% (4.5 mg/ml) sodium chloride and 5% (50 mg/ml) glucose injectable solution
- 0.9% (9 mg/ml) sodium chloride and 5% (50 mg/ml) glucose injectable solution
- 0.18% (1.8 mg/ml) sodium chloride and 4% (40 mg/ml) glucose injectable solution
- 10% (100 mg/ml) glucose injectable solution
- 10% (100 mg/ml) dextran 40 in 0.9% (9 mg/ml) sodium chloride injectable solution
- 10% (100 mg/ml) dextran 40 in 5% (50 mg/ml) glucose injectable solution
- 6% (60 mg/ml) dextran 70 in 0.9% (9 mg/ml) sodium chloride injectable solution
- 6% (60 mg/ml) dextran 70 in 5% (50 mg/ml) glucose injectable solution
Ceftazidime at concentrations between 0.05 mg/ml (0.005%) and 0.25 mg/ml (0.025%) is compatible with peritoneal dialysis fluid (lactate).
Ceftazidime can be reconstituted for intramuscular use with 0.5% (5 mg/ml) or 1% (10 mg/ml) lidocaine hydrochloride injectable solution, with the concentrations detailed in Table 1.
The contents of a 500 mg vial of Ceftazidima Qilu, reconstituted with 1.5 ml of water for injectable preparations, can be added to a metronidazole injection (500 mg in 100 ml), in which both will retain their activity.
500 mg powder for injectable solution, 1 g, 2 g powder for injectable solution or perfusion:
THIS SECTION SHOULD BE PRESENTED WITH PICTOGRAMS, AS IN THE PREVIOUS MATERIAL.
Preparation of solutions for bolus injection:
- Insert the needle of the syringe through the vial closure and inject the recommended volume of diluent. Remove the needle from the syringe.
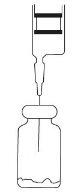
- Shake until dissolved: carbon dioxide is released and a transparent solution is obtained in 1 or 2 minutes.
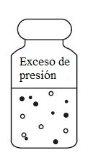
- Invert the vial. With the syringe plunger fully compressed, insert the needle through the vial closure and withdraw the total volume of the solution into the syringe (the pressure created in the vial may aid in withdrawal). Ensure the needle remains in the solution and not in the upper free space. The withdrawn solution may contain small carbon dioxide bubbles that can be ignored.
These ceftazidime solutions can be administered directly into the vein or introduced into the tube of an enteral nutrition system in case the patient is receiving parenteral fluids. Ceftazidime is compatible with the intravenous fluids listed above.
1 g, 2 g powder for injectable solution and perfusion:
Preparation of i.v. perfusion solutions of Ceftazidima Qilu in usual vial presentations (minibag or burette-type perfusion system):
Prepare with a total of 50 ml (for 1 g and 2 g vials) of compatible diluent (from those listed above), added in TWO stages as explained below.
- Insert the needle of the syringe through the vial closure and inject 10 ml of diluent for 1 g and 2 g vials.
- Remove the needle and shake the vial until a clear solution is obtained.
- Do not insert a needle to release gas until the product is dissolved. Insert a needle to release gas through the vial closure to release the internal pressure.
- Transfer the reconstituted solution to the final distribution vehicle (e.g., minibag or burette-type perfusion system) to a total volume of at least 50 ml and administer by intravenous perfusion over 15-30 minutes.
Note: To maintain the sterility of the product, it is important that the needle for gas release is not inserted through the vial closure until the product is dissolved.
Any unused antibiotic solution should be discarded.
For single use.
Disposal of unused medicinal product and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CEFTAZIDIME QILU 2 g POWDER FOR INJECTION AND INFUSION SOLUTIONDosage form: INJECTABLE, 1 gActive substance: ceftazidimeManufacturer: Fresenius Kabi España, S.A.U.Prescription requiredDosage form: INJECTABLE, 2000 mgActive substance: ceftazidimeManufacturer: Fresenius Kabi España, S.A.U.Prescription requiredDosage form: INJECTABLE, 1 gActive substance: ceftazidimeManufacturer: Ldp Laboratorios Torlan S.A.Prescription required
Online doctors for CEFTAZIDIME QILU 2 g POWDER FOR INJECTION AND INFUSION SOLUTION
Discuss questions about CEFTAZIDIME QILU 2 g POWDER FOR INJECTION AND INFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















