
CANESPIE BIFONAZOL 10 mg/g CREAM

How to use CANESPIE BIFONAZOL 10 mg/g CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Canespie Bifonazol 10 mg/g Cream
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 7 days.
Contents of the Package Leaflet:
- What is Canespie Bifonazol and what is it used for
- What you need to know before using Canespie Bifonazol
- How to use Canespie Bifonazol
- Possible side effects
- Storage of Canespie Bifonazol
- Package Contents and Additional Information
1. What is Canespie Bifonazol and what is it used for
This medication belongs to the group of medications called antifungals (medications used to treat infections caused by fungi and yeasts).
It is indicated for the treatment of "athlete's foot" (superficial skin infection caused by fungi and located between the folds of the toes) in adults and adolescents from 12 years of age.
The main symptoms of athlete's foot are: itching, redness (erythema), cracks between the toes, peeling that can lead to inflammation or pustules. It only appears on the feet. It usually starts between the toes, but can also spread to the sole and sides of the feet.
You should consult a doctor if your symptoms worsen or do not improve after 7 days.
2. What you need to know before starting to use Canespie Bifonazol
Do not use Canespie Bifonazol
- if you are allergic to bifonazol, imidazoles in general, or any of the other components of this medication (listed in section 6).
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Canespie Bifonazol.
- This medication is for external use only. Avoid contact with the eyes and mucous membranes, and if it occurs, rinse with cold water,
- do not ingest,
- it is not recommended to cover the affected area with bandages after applying the medication, as it promotes systemic absorption.
Children and Adolescents
Do not administer this medication to children under 12 years of age.
For adolescents from 12 years of age, see the section How to use Canespie Bifonazolbelow.
Using Canespie Bifonazol with other medications
Inform your doctor or pharmacist if you are using or have recently used other medications or may need to take any other medication.
If you are being treated with warfarin (oral anticoagulant), it may be necessary to modify your dose, as the effects of this medication may be affected by the use of bifonazol.
It is not recommended to use other medications on the same areas where this medication is applied at the same time.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy
It is not recommended to use this medication during pregnancy or in women of childbearing age who are not using contraceptive methods.
Breastfeeding
Caution should be exercised during breastfeeding, as bifonazol may be excreted in breast milk. If administered, breastfeeding should be interrupted and replaced.
Driving and Using Machines
The influence of this medication on the ability to drive and use machines is nil or insignificant.
Canespie Bifonazol contains cetyl alcohol and benzyl alcohol
This medication may cause local skin reactions (such as contact dermatitis) because it contains cetyl alcohol.
This medication contains 2 mg of benzyl alcohol per gram of cream. Benzyl alcohol may cause allergic reactions and moderate local irritation.
3. How to use Canespie Bifonazol
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults and adolescents from 12 years of age:
1 application to the affected area once a day, preferably before bedtime. The duration of treatment is 3 weeks.
If after 7 days of use you do not observe an improvement in your symptoms, consult your doctor.
Method of administration:
This medication is administered topically.
Apply a sufficient amount of cream to completely cover the affected area, paying special attention to the folds between the toes, and rub until fully absorbed. It is recommended to wash your hands after each application.
Package with Applicator
For the package with the incorporated applicator, follow these instructions:
- Before applying the cream, clean and dry the feet well, especially between the toes.
- Remove the cap from the applicator.
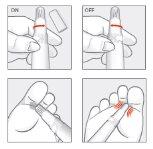
- To open the tube, turn the base of the applicator to the ON position.

- Press the tube until the cream comes out.
- Apply and spread a thin layer of cream to the affected area of the foot and/or between the toes. Gently rub with the help of the soft part of the applicator and let dry.
- To close the tube, turn the base of the applicator to the OFF position.
- Clean the outside of the applicator with a damp cloth, making sure no cream residue remains. Do not remove the soft part of the applicator. Do not use detergents or chemical products to clean the applicator.
- Cap the applicator with the cap.
If you use more Canespie Bifonazol than you should
If you apply more cream than your pharmacist has indicated, you may feel a certain burning sensation, redness, or swelling that will disappear after stopping treatment.
This medication should not be ingested. If accidentally ingested, contact your doctor.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or go to a medical center immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Canespie Bifonazol
If you forget to use this medication when you should, apply the cream as soon as you remember and continue with your usual treatment regimen. Do not use a double dose to make up for forgotten doses.
If you interrupt treatment with Canespie Bifonazol
Do not stop treatment before the indicated time, as irregular use or premature interruption of treatment carries the risk of relapse.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The following side effects have been reported, whose frequency cannot be estimated from the available data (unknown frequency).
Contact dermatitis, allergic dermatitis, erythema, itching, exanthema, urticaria, blisters, skin exfoliation, eczema, dry skin, skin irritation, skin maceration, burning sensation on the skin, pain at the application site, and peripheral edema at the application site.
Reporting Side Effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Canespie Bifonazol
Keep this medication out of the sight and reach of children.
Store in the original packaging. No special storage conditions are required.
Package with Applicator (15 g):
After the first opening, the cream is stable for 6 months.
Package without Applicator (20g):
After the first opening, the cream is stable for 6 months. Once the package is opened, do not store above 25°C.
Do not use Canespie Bifonazol after the expiration date shown on the package, after EXP. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packages and medications you no longer need at the Sigre Point in the pharmacy. If in doubt, ask your pharmacist how to dispose of packages and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Canespie Bifonazol
- The active ingredient is bifonazol. Each gram of cream contains 10 mg of bifonazol.
- The other ingredients (excipients) are: benzyl alcohol, cetyl alcohol, cetyl palmitate, octyldodecanol, polysorbate 60, sorbitan stearate, and purified water.
Appearance of the Product and Package Contents
Canespie Bifonazol is a white, odorless cream.
Package with Applicator (15 g): It is presented in a cardboard box containing a polyethylene/aluminum tube with 15 g of cream and an incorporated applicator.
Package without Applicator (20 g): It is presented in a cardboard box containing an aluminum tube with a blind mouth and an HDPE screw cap containing 20 g of cream.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Bayer Hispania, S.L.
Av. Baix Llobregat, 3 – 5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
Kern Pharma, S.L.
Polígono Industrial Colón II
Venus, 72
08228 Terrassa (Barcelona)
Spain
GP Grenzach Produktions GmbH
Emil-Barell-Str. 7
D-79639 Grenzach-Wyhlen
Germany
Date of Revision of this Package Leaflet:
June 2019
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CANESPIE BIFONAZOL 10 mg/g CREAMDosage form: CREAM, 1% bifonazoleActive substance: bifonazoleManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: TOPICAL SOLUTION, 1 g bifonazole/100 mlActive substance: bifonazoleManufacturer: Bayer Hispania S.L.Prescription not requiredDosage form: CREAMActive substance: bifonazoleManufacturer: Laboratorios Ern S.A.Prescription required
Online doctors for CANESPIE BIFONAZOL 10 mg/g CREAM
Discuss questions about CANESPIE BIFONAZOL 10 mg/g CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








