
BUTYLSCOPOLAMINE KALCEKS 20 mg/ml INJECTABLE SOLUTION

How to use BUTYLSCOPOLAMINE KALCEKS 20 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Butilescopolamina Kalceks 20mg/ml solution for injection EFG
butilescopolamine bromide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Butilescopolamina Kalceks and what is it used for
- What you need to know before you are given Butilescopolamina Kalceks
- How Butilescopolamina Kalceks is given
- Possible side effects
- Storage of Butilescopolamina Kalceks
- Contents of the pack and other information
1. What is Butilescopolamina Kalceks and what is it used for
Butilescopolamina Kalceks contains the active substance butilescopolamine bromide. This belongs to a group of medicines called antispasmodics. These medicines relieve spasms (contractions similar to cramps) of internal organs and relieve the pain that comes from them.
This medicine is used to relieve spasms of the smooth muscles of the digestive and genitourinary tracts (stomach, intestines, bile ducts, pancreas, and urinary tract).
Butilescopolamina Kalceks may also be used in medical diagnostic procedures.
2. What you need to know before you are given Butilescopolamina Kalceks
Do not useButilescopolamina Kalceks
- if you are allergic to butilescopolamine bromide or any of the other ingredients of this medicine (listed in section 6)
- if you have glaucoma (eye disease)
- if you have an enlarged prostate and have difficulty or pain when urinating
- if you have intestinal obstruction
- if you have an abnormally enlarged intestine (megacolon)
- if you have an increased heart rate
- if you have a disease called myasthenia gravis (characterized by extreme muscle weakness).
You should not receive any injection of butilescopolamine bromide into the muscle if you are taking medicines to prevent blood clot formation (anticoagulants), as a hematoma (bruise) may occur.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using this medicine:
- if you have abdominal pain of unknown origin that persists or worsens, or appears with symptoms such as fever, nausea, vomiting, changes in bowel movements, abdominal tenderness, decreased blood pressure, fainting, or blood in the stool
- if the intestine stops working properly (intestinal atony)
- if you have esophageal inflammation associated with acid reflux (when stomach acid rises and enters the esophagus)
- if you have severe colon inflammation that recurs frequently (ulcerative colitis)
- if you have liver or kidney problems
- if you have hyperthyroidism (when the thyroid gland produces too many thyroid hormones)
- if you have chronic bronchitis (inflammation of the bronchi).
You should go to the doctor immediately, if after the injection of butilescopolamine bromide, pain and redness of an eye with vision loss appear. This may be a sign of increased pressure inside the eye due to a previously undiagnosed and untreated narrow-angle glaucoma.
Allergic reactions have been observed after the injection of butilescopolamine bromide (see section 4). Therefore, you will be monitored after the injection of butilescopolamine bromide and treated accordingly if such reactions occur.
Other medicines and Butilescopolamina Kalceks
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription, including herbal medicines.
Especially tell your doctor or pharmacist if you are taking any of the following:
- medicines for treating depression called tricyclic or tetracyclic antidepressants
- medicines for treating allergies (antihistamines)
- medicines for treating mental illnesses
- medicines for treating heart failure or asthma (betamimetics)
- medicines for treating heart rhythm disorders (quinidine or disopyramide)
- amantadine (medicine for the treatment of Parkinson's disease)
- medicines for treating respiratory disorders (such as tiotropium, ipratropium, and atropine-like substances)
- metoclopramide (used to treat nausea, vomiting, or gastrointestinal disorders).
If you are not sure if any of the above situations apply to you, talk to your doctor or pharmacist before you are given this medicine.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before you are given this medicine.
Pregnancy
There is limited experience with the use of this product in pregnant or breast-feeding women. Consequently, for safety reasons, the use of this medicine during pregnancy is not recommended.
During pregnancy, the medicine should only be used under the advice of a doctor who will evaluate the risk/benefit ratio.
Breast-feeding
During breast-feeding, the medicine should only be used under the advice of a doctor who will evaluate the risk/benefit ratio.
Driving and using machines
Some people may experience vision changes and dizziness after being treated with this medicine. If you experience these effects, do not drive or use machines until your vision returns to normal or you stop feeling dizzy.
Butilescopolamina Kalceks contains sodium
This medicine contains less than 23 mg of sodium (1 mmol); this is essentially "sodium-free".
3. How Butilescopolamina Kalceks is given
Butilescopolamina Kalceks will be given to you by a doctor or nurse as a slow injection into a vein, into a muscle, or under the skin. The dose will be determined by your doctor.
This medicine should not be given continuously on a daily basis or for prolonged periods without investigating the cause of abdominal pain.
Adults and children over 12years
The dose is 20 to 40 mg (1-2 ampoules) given several times a day. The maximum daily dose is 100 mg (5 ampoules).
Use in children
In severe cases in infants and children, the dose is 0.3 to 0.6 mg/kg body weight, given several times a day. The maximum daily dose should not exceed 1.5 mg/kg body weight.
If you have been given too muchButilescopolamina Kalceks
If you think you have been given too much, tell your doctor or nurse immediately. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or go to a medical center, or call the Toxicology Information Service, Tel. 91 562 04 20 indicating the medicine and the amount taken.
The following symptoms may occur: dry mouth, skin redness, difficulty urinating, rapid heartbeat, and vision disorders.
If you miss a dose of Butilescopolamina Kalceks
You will not receive a double dose to make up for the missed dose. You will only receive the next dose if necessary according to your health status.
If you stop using Butilescopolamina Kalceks
Your doctor will give you an injection only in acute cases. If it is necessary to continue treatment, your doctor will switch you to butilescopolamine bromide in tablets.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Many of the adverse reactions can be associated with the anticholinergic properties of butilescopolamine bromide, which are usually mild and transient.
Adverse reactions have been tabulated using the following frequency convention:
Very common may affect more than 1 in 10 people
Common may affect up to 1 in 10 people
Uncommon may affect up to 1 in 100 people
Rare may affect up to 1 in 1,000 people
Very rare may affect up to 1 in 10,000 people
Frequency not known cannot be estimated from the available data
Disorders of the immune system
Frequency not known: anaphylactic shock (severe and sudden allergic reaction, which manifests with difficulty breathing, insufficient blood circulation, and swelling, and can be fatal), anaphylactic reactions, difficulty breathing, skin reactions (such as hives, rash, skin redness, itching), other hypersensitivity reactions.
Psychiatric disorders
Frequency not known: mental confusion in elderly people, excitability, irritability.
Eye disorders
Common:disorders of visual accommodation (focusing).
Frequency not known: pupil dilation, increased pressure inside the eye, reduced tear secretion.
Cardiac disorders
Common:increased heart rate.
Frequency not known: palpitations.
Vascular disorders
Common:dizziness.
Frequency not known: decreased blood pressure, flushing.
Respiratory disorders
Frequency not known: thickening of bronchial secretions.
Gastrointestinal disorders
Common:dry mouth.
Frequency not known: constipation.
Disorders of the skin and subcutaneous tissue
Frequency not known: abnormal sweating.
Renal and urinary disorders
Frequency not known: difficulty urinating.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Health Products Agency (AEMPS) website (http://www.aemps.gob.es/). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Butilescopolamina Kalceks
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the carton and on the ampoule after EXP. The expiry date is the last day of the month shown.
Shelf-life after opening of the ampoule: The administration of the medicine should take place immediately.
Shelf-life after dilution: The chemical and physical stability in use has been demonstrated for 24 hours at 25°C and 2-8°C.
From a microbiological point of view, the product should be used immediately, unless the method of opening/dilution precludes the risk of microbial contamination. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Butilescopolamina Kalceks
- The active substance is butilescopolamine bromide.
Each ampoule (1 ml) contains 20 mg of butilescopolamine bromide.
- The other ingredients are sodium chloride, concentrated hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment), water for injections.
Appearance of Butilescopolamina Kalceks and pack contents
Solution for injection (injectable).
Clear, colorless or almost colorless solution, without visible particles.
1 ml glass ampoules of type I.
The ampoules are placed in a PVC tray. The trays are packaged in cardboard boxes.
Package sizes: 5 or 10 ampoules
Not all pack sizes may be marketed.
Marketing authorization holder
AS KALCEKS
Krustpils iela 71E, Riga, LV-1057,
Latvia
Tel.: +371 67083320
E-mail: [email protected]
Manufacturer
Akciju sabiedriba “Kalceks”
Krustpils iela 71E, Riga, LV-1057, Latvia
You can request moreinformation about this medicine from the local representative of the marketing authorization holder
EVER Pharma Therapeutics Spain SL
c/ Toledo 170
28005 Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Czech Republic Butylskopolaminium bromid Kalceks
Austria Butylscopolaminiumbromid Kalceks 20 mg/ml Injektionslösung
Belgium Scopolamine butylbromide Kalceks 20 mg/ml solution injectable
Scopolamine butylbromide Kalceks 20 mg/ml oplossing voor injectie
Scopolamine butylbromide Kalceks 20 mg/ml Injektionslösung
Bulgaria Scopolamine butylbromide Kalceks 20 ??/?? ??????????? ???????
France SCOPOLAMINE BUTYLBROMURE KALCEKS 20 mg/mL, solution injectable
Italy Scopolamina butilbromuro Kalceks
Latvia Hyoscine butylbromide Kalceks 20 mg/ml škidums injekcijam
Poland Scopolamine butylbromide Kalceks
Portugal Butilescopolamina Kalceks
Norway Skopolaminbutylbromid Kalceks
Slovakia Scopolamine butylbromide Kalceks 20 mg/ml injekcný roztok
Spain Butilescopolamina Kalceks 20 mg/ml solución inyectable EFG
Sweden Hyoscine butylbromide Kalceks
Netherlands Scopolamine butylbromide Kalceks 20 mg/ml oplossing voor injectie
Date of last revision of this leaflet:October 2022.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Method of administration
Intravenous, intramuscular, or subcutaneous injection.
Instructions for use, disposal, and other handling
For single use. Once opened, the unused solution should be discarded.
The medicine should be visually inspected before use. It should only be used if the solution is clear and free of particles.
It can be used diluted with dextrose or with a 0.9% sodium chloride injection solution.
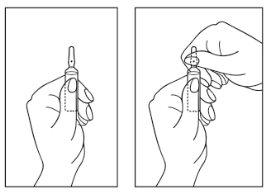
Instructions for opening the ampoule:
- Turn the ampoule with the colored point upwards. If some solution remains in the top part of the ampoule, gently tap with your finger to make the solution go down to the bottom of the ampoule.
- Use both hands to open; while holding the bottom of the ampoule with one hand, separate the top part of the ampoule in the opposite direction to the colored point (see the images below).
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Average pharmacy price4.06 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUTYLSCOPOLAMINE KALCEKS 20 mg/ml INJECTABLE SOLUTIONDosage form: TABLET, 10 mg butylscopolamineActive substance: butylscopolamineManufacturer: Opella Healthcare Spain S.L.Prescription not requiredDosage form: INJECTABLE, 20 mg butylscopolamine bromide/ mlActive substance: butylscopolamineManufacturer: Opella Healthcare Spain S.L.Prescription requiredDosage form: TABLET, 10 mgActive substance: butylscopolamineManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for BUTYLSCOPOLAMINE KALCEKS 20 mg/ml INJECTABLE SOLUTION
Discuss questions about BUTYLSCOPOLAMINE KALCEKS 20 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















