
BUDESONIDE/FORMOTEROL CIPLA 320 mcg/9 mcg INHALATION POWDER (SINGLE DOSE)

How to use BUDESONIDE/FORMOTEROL CIPLA 320 mcg/9 mcg INHALATION POWDER (SINGLE DOSE)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Budesonide/Formoterol Cipla 320 micrograms/9 micrograms/inhalation, powder for inhalation (single-dose)
Budesonide/formoterol fumarate dihydrate
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Budesonide/Formoterol Cipla and what is it used for
- What you need to know before taking Budesonide/Formoterol Cipla
- How to use Budesonide/Formoterol Cipla
- Possible side effects
5 Conservation of Budesonide/Formoterol Cipla
- Package contents and additional information
1. What is Budesonide/Formoterol Cipla and what is it used for
Budesonide/Formoterol Cipla is an inhaler used for the treatment of asthma in adults and adolescents between 12 and 17 years of age. It is also used for the symptomatic treatment of Chronic Obstructive Pulmonary Disease (COPD) in adults over 18 years of age. It contains two different medications: budesonide and formoterol fumarate dihydrate.
- Budesonide belongs to a group of medications called "corticosteroids", and it works by reducing and preventing inflammation in your lungs.
- Formoterol fumarate dihydrate belongs to a group of medications called "long-acting beta2 adrenergic agonists" or "bronchodilators", and it works by relaxing the muscles of the airways, making it easier for you to breathe.
Asthma
Your doctor will prescribe two different asthma inhalers for you: Budesonide/Formoterol Cipla and another separate "reliever" inhaler.
- Use Budesonide/Formoterol Cipla daily, as it helps prevent the onset of asthma symptoms.
- Use your "reliever" inhaler when you experience asthma symptoms, to facilitate breathing.
Do not use Budesonide/Formoterol Cipla as a "reliever" inhaler.
Chronic Obstructive Pulmonary Disease (COPD)
Budesonide/Formoterol Cipla can also be used to treat COPD symptoms in adults. COPD is a chronic disease of the pulmonary airways, usually caused by smoking.
2. What you need to know before taking Budesonide/Formoterol Cipla
Do not use Budesonide/Formoterol Cipla:
if you are allergic to budesonide, formoterol, or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Budesonide/Formoterol Cipla if:
- you have diabetes,
- you have a lung infection,
- you have high blood pressure, or have ever had any heart disease (including irregular heartbeats, rapid pulse, narrowing of the arteries, or heart failure),
- you have thyroid or adrenal gland problems,
- you have low potassium levels in your blood,
- you have severe liver problems.
Consult your doctor if you experience blurred vision or other visual problems.
Other medications and Budesonide/Formoterol Cipla
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
In particular, tell your doctor or pharmacist if you are using any of the following medications:
- beta-blocker medications (such as atenolol or propranolol for high blood pressure), including eye drops (such as timolol for glaucoma),
- medications to treat rapid or irregular heartbeat (such as quinidine, disopyramide, procainamide),
- Medications to treat allergies, also called antihistamines, such as terfenadine.
- Oxytocin, a medication to facilitate childbirth.
- Procarbazine, a medication to treat cancer.
- Medications such as digoxin, commonly used to treat heart failure.
- Diuretics (such as furosemide), used to treat high blood pressure.
- Corticosteroids (such as prednisolone). These are used to treat inflammation or prevent organ transplant rejection.
- Xanthine medications (such as theophylline or aminophylline). These are often used to treat asthma. Other bronchodilators (such as salbutamol).
- Other medications to widen the airways, also called bronchodilators (such as salbutamol).
- Medications to treat depression, also called tricyclic antidepressants (such as amitriptyline) and the antidepressant nefazodone.
- Medications to treat mental disorders, nausea, or vomiting, called phenothiazine medications (such as chlorpromazine and prochlorperazine).
- Medications to treat fungal infections (such as ketoconazole, itraconazole, voriconazole, posaconazole) and bacterial infections (such as clarithromycin and telithromycin, furazolidone).
- Medications for Parkinson's disease (such as levodopa).
- Medications for thyroid problems (such as levothyroxine).
- Medications called "HIV protease inhibitors" (such as ritonavir, cobicistat) to treat HIV infections. The effects of Budesonide/Formoterol Cipla may increase, and your doctor may want to monitor you closely.
If you are in any of these situations, or if you are unsure, ask your doctor or pharmacist before using Budesonide/Formoterol Cipla.
Also, inform your doctor or pharmacist if you are going to undergo general anesthesia for surgery or dental treatment.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or plan to become pregnant, consult your doctor before using Budesonide/Formoterol Cipla, unless your doctor advises you to do so.
- If you become pregnant during treatment with Budesonide/Formoterol Cipla, do not stop using it and consult your doctor immediately.
- If you are breastfeeding, consult your doctor before using Budesonide/Formoterol Cipla.
Driving and using machinery
The influence of Budesonide/Formoterol Cipla on the ability to drive and use machinery is negligible.
Budesonide/Formoterol Cipla contains lactose
This medication contains lactose. It may cause allergic reactions in patients with cow's milk protein allergy. If your doctor has told you that you have an intolerance to certain sugars, consult with them before using this medication.
3. How to use Budesonide/Formoterol Cipla
- Follow your doctor's instructions for using this medication exactly. If you are unsure, consult your doctor or pharmacist again.
- It is essential to use Budesonide/Formoterol Cipla daily, even if you do not have symptoms of asthma or COPD at that time.
- If you are using Budesonide/Formoterol Cipla for asthma, your doctor will want to check your symptoms regularly.
If you have been taking oral corticosteroid tablets for asthma or COPD, your doctor may reduce the number of tablets you take once you start using Budesonide/Formoterol Cipla. If you have been taking oral corticosteroid tablets for a long time, your doctor may ask you to have blood tests from time to time. You may feel unwell when your oral corticosteroid dose is reduced, even if your lung symptoms are improving. You may experience symptoms such as nasal congestion or runny nose, weakness or pain in the joints or muscles, and skin rash (hives). If you are concerned about any of these symptoms, or if you experience symptoms such as headache, fatigue, nausea, or vomiting, contact your doctor immediately. You may need to take another medication if you develop allergic or arthritic symptoms. You should consult your doctor if you are unsure about how to continue using Budesonide/Formoterol Cipla.
Your doctor may consider adding oral corticosteroid tablets to your regular treatment during periods of stress (e.g., when you have a chest infection or before surgery).
Important information about asthma or COPD symptoms
If, while using Budesonide/Formoterol Cipla, you experience difficulty breathing or wheezing, you should continue using Budesonide/Formoterol Cipla but contact your doctor as soon as possible, as you may need additional treatment.
Contact your doctor immediately if:
- Your breathing is getting worse or you wake up at night with asthma symptoms.
- You start to feel chest tightness in the morning, or the chest tightness lasts longer than usual. These signs may indicate that your asthma or COPD is not adequately controlled, and you may need immediate different or additional treatment.
Asthma
Use Budesonide/Formoterol Cipla daily, as it helps prevent the onset of asthma symptoms.
Adults (from 18 years)
- The usual dose is 1 inhalation, twice a day.
- Your doctor may increase the dose to a maximum of 2 inhalations, twice a day.
- If your symptoms are well-controlled, your doctor may ask you to use the medication once a day.
Adolescents (from 12 to 17 years of age)
- The usual dose is 1 inhalation, twice a day.
- If your symptoms are well-controlled, your doctor may ask you to use the medication once a day.
Budesonide/Formoterol Cipla is not recommended for use in children under 12 years of age.
Your doctor (or nurse) will help you treat your asthma. They will adjust the dose of this medication to the lowest dose that controls your asthma. However, do not adjust the dose without talking to your doctor or nurse first
Use your other "reliever" inhaler to treat asthma symptoms. Always carry this "reliever" inhaler with you to use when needed. Do not use Budesonide/Formoterol Cipla to treat asthma symptoms, but use your "reliever" inhaler instead.
Chronic Obstructive Pulmonary Disease (COPD)
Use only in adults (over 18 years).
- The usual dose is 1 inhalation, twice a day.
Your doctor may also prescribe other bronchodilator medications, such as anticholinergics (e.g., ipratropium bromide or tiotropium bromide), for your COPD.
Instructions for use:
Your doctor, nurse, or pharmacist should teach you how to use the inhaler and review it periodically to ensure you are using it correctly.
The inhaler contains 60 doses of medication in powder form in a rolled-up aluminum strip. It has a dose counter that shows how many doses are left, counting down from 60 to 0. When the last 10 doses are reached, the numbers appear on a red background.
The inhaler is not refillable; it must be discarded when empty and replaced with a new one.
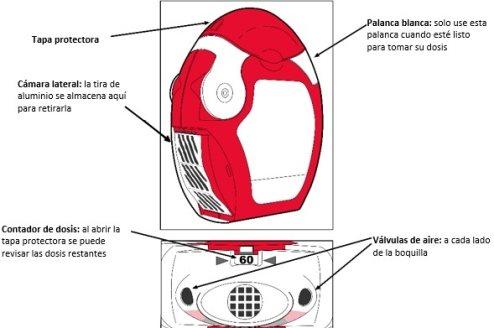
Before using the inhaler:
- The transparent lateral chamber of the inhaler must be opened.
- The aluminum strip must be cut from the lateral chamber by carefully pulling the strip against the "teeth" of the lateral chamber as shown below. Do not pull the strip forcefully or tear it.
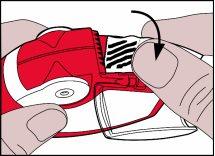
- Close the lateral chamber cover and discard the used strip.
Important:
As the inhaler is used, the lateral chamber will gradually fill with the used aluminum strip. The aluminum strips with black lines do not contain medication. Eventually, the numbered sections of the strip will appear in the lateral chamber.
There should never be more than 2 sections of aluminum stripin the lateral chamber, as this can cause the inhaler to jam. The excess strip should be carefully cut as indicated above and left in a safe place.
Using the inhaler:
Hold the inhaler with your hands, as shown in the images.
- Opening
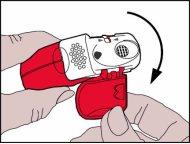
- The protective cap must be opened downwardsto show the mouthpiece.
- Check the dose counter to see how many doses are left.
- Dose preparation
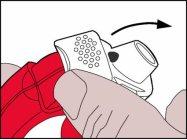
- The white levermust be raised upwards. Make sure the lateral chamber is closed.
Remember:The white lever should only be manipulated when the patient is ready to inhale their dose of medication. If the patient plays with the white lever, they may waste doses.
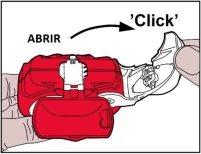
- Opening:The white levermust be fully openeduntil it clicks. This action moves a new dose to its position with its corresponding number on top in the counter
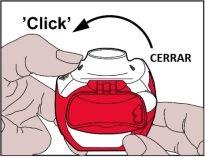
- Closing:Then, the white levermust be fully closeduntil it clicks again in its original position. This way, the inhaler is ready for immediate use.
- Inhaling the dose
- With the inhaler mouthpiece away from your mouth, you must exhale as much as you can until you feel comfortable. Never exhale directly intothe inhaler, as this may affect the dose.
- The inhaler must be held with the protective cap facing downwards.
- Close your lips firmly around the mouthpiece.
- You must inhaleas deeplyand as forcefullyas possible through the inhaler, without breathing through your nose.
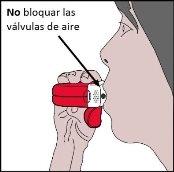
- Then, remove the inhaler from your mouth and hold your breath for 5 to 10 secondsor as long as you can without discomfort.
- Then, start breathing slowly, but not into the inhaler.
- Close the mouthpiece protective cap.
- Rinse your mouth with water, which you should then spit out. This may help prevent fungal infections in the mouth and avoid hoarseness.
Cleaning
- If necessary, the outside of the mouthpiece can be cleaned with a dry cloth.
- Never separate the parts of the inhaler to clean them or for any other purpose!
- The parts of the inhaler cannot be cleaned with water or wet cloths, as moisture may affect the dose!
- Never insert pins or any other sharp object into the mouthpiece or any other part, as this can damage your inhaler!
If you use more Budesonide/Formoterol Cipla than you should
It is essential to use the dose indicated in the leaflet or prescribed by your doctor. Do not increase your dose without consulting your doctor.
The most common symptoms and signs that may occur after using more Budesonide/Formoterol Cipla than you should are tremors, headache, and rapid heartbeat.
If you have used more Budesonide/Formoterol Cipla than you should, consult your doctor, nurse, or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount used.
If you forget to use Budesonide/Formoterol Cipla
- If you forget a dose, take it as soon as you remember. However, if it is almost time for your next dose, do not worry about the missed dose.
- Do notuse a double dose to make up for missed doses.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you experience any of the following situations, stop using Budesonide/Formoterol Cipla and consult your doctor immediately:
- Your face swells, particularly around the mouth (tongue and/or throat and/or difficulty swallowing) or hives along with breathing difficulties (angioedema) and/or a sudden feeling of fainting, indicating that you may be suffering from an allergic reaction. This occurs rarely, (it can affect up to 1 in 1,000 patients).
- You experience acute wheezing or difficulty breathing immediately after using your inhaler. If you experience any of these symptoms, stop using Budesonide/Formoterol Cipla immediately and use your "reliever inhaler". Contact your doctor immediately as you may need to change your treatment.This occurs very rarely, (it can affect up to 1 in 10,000 patients).
Other possible adverse effects:
Frequent (may affect up to 1 in 10 people)
- Palpitations (noticing heartbeats), tremors. When these effects appear, they are usually mild and disappear when continuing to use Budesonide/Formoterol Cipla.
- Thrush (fungal infection) in the mouth. This effect is less likely if you rinse your mouth with water after using Budesonide/Formoterol Cipla.
- Mild throat irritation, cough, hoarseness.
- Headache.
- Pneumonia (lung infection) in patients with COPD.
Inform your doctor if you experience any of the following symptoms while inhaling Budesonide/Formoterol Cipla, as they could be symptoms of a lung infection:
- Fever or chills,
- Increased mucus production, change in mucus color,
- Increased cough or increased breathing difficulties.
Infrequent (may affect up to 1 in 100 people)
- Aggressiveness.
- Anxiety.
- Restlessness, nervousness, or agitation.
- Difficulty sleeping.
- Dizziness.
- Nausea (discomfort).
- Accelerated heart rate.
- Bruises on the skin.
- Muscle cramps.
- Blurred vision.
Rare (may affect up to 1 in 1,000 people)
- Rash, itching.
- Bronchospasm (contraction of the airway muscles, causing wheezing). If wheezing occurs suddenly just after using Budesonide/Formoterol Cipla, stop using it and consult your doctor immediately.
- Low potassium levels in the blood.
- Irregular heartbeat.
Very rare (may affect up to 1 in 10,000 people)
- Depression.
- Changes in behavior, especially in children.
- Chest pain or tightness (angina pectoris).
- Increased blood sugar (glucose) levels.
- Taste disturbances, such as unpleasant mouth taste.
- Changes in blood pressure.
- Weight gain, moon face, weakness, abdominal obesity (Cushing's syndrome).
Inhaled corticosteroids may affect the normal production of steroid hormones in the body, especially if high doses are used for a long time. These effects include:
- changes in bone mineral density (decrease in bone mass),
- cataracts (loss of lens transparency in the eye),
- glaucoma (increased eye pressure),
- growth delay in children and adolescents,
- effects on the adrenal glands (small glands located next to the kidneys),
- Cushingoid features,
- it may also occur an increased susceptibility to infections and a deterioration of the ability to adapt to stress.
These effects are much less likely with inhaled corticosteroids than with corticosteroid tablets.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Budesonide/Formoterol Cipla
Keep this medicine out of sight and reach of children.
Do not store at a temperature above 30 °C.
Do not use this medicine after the expiration date that appears on the packaging or label of the inhaler after CAD. The expiration date is the last day of the month indicated.
Medicines should not be thrown away through the sewers or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Budesonide/Formoterol Cipla
- The active ingredients are budesonide and formoterol fumarate dihydrate. Each inhaled dose contains 160 micrograms of budesonide and 4.5 micrograms of formoterol fumarate dihydrate, which corresponds to a measured dose (a single dose contained in the blister) of 194.7 micrograms of budesonide and 6.1 micrograms of formoterol fumarate dihydrate.
- The other component is lactose monohydrate (which contains milk proteins).
Appearance of the product and package contents
Budesonide/Formoterol Cipla is a red/white plastic inhaler that contains your medicine. Each inhaler contains a OPA/Al/PVC-Al blister with 60 pre-measured doses of mixed powder. The inhaled powder is white to off-white or slightly yellowish without agglomerates.
Budesonide/Formoterol Cipla is available in packages of 1, 2, 6 inhalers, each containing 60 doses.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Cipla Europe NV
De Keyserlei 60C, Bus-1301
2018 Antwerp
Belgium
Manufacturer
AEROPHARM GmbH
François-Mitterrand-Allee 1
07407 Rudolstadt
Germany
or
Lek Pharmaceuticals d.d.
Verovškova ulica 57
1526 Ljubljana
Slovenia
or
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben, Saxony-Anhalt
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Cipla Europe NV branch in Spain,
C/Guzmán el Bueno, 133 Edif Britannia-28003- Madrid
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium:Airbufo Forspiro 160 micrograms/4.5 micrograms/inhalation, inhalation powder, pre-dispensed
Denmark: AirBuFo Forspiro
Finland: AEROCOMP Forspiro 160 micrograms/4.5 micrograms/dose, inhalation powder, pre-dispensed
France: AirBuFo Forspiro 160 micrograms/4.5 micrograms/dose, powder for inhalation in a single-dose container
Ireland: AirBuFo Forspiro 160 micrograms/4.5 micrograms/dose inhalation powder, pre-dispensed
Italy: AirBuFo Forspiro
Norway: AirBuFo Forspiro
Portugal: AirBuFo Forspiro
Sweden: Airbufo Forspiro 160 micrograms/4.5 micrograms/dose, inhalation powder, pre-dispensed
Date of the last revision of this prospectus: February 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
You can access detailed and updated information on how to administer this medicine by scanning the QR code included in the packaging with your mobile phone (smartphone). You can also access this information at the following internet address: https://cima.aemps.es/info/85833.
- Country of registration
- Average pharmacy price39.56 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUDESONIDE/FORMOTEROL CIPLA 320 mcg/9 mcg INHALATION POWDER (SINGLE DOSE)Dosage form: PULMONARY INHALATION, 160 micrograms / 4.5 microgramsActive substance: formoterol and budesonideManufacturer: Teva Pharma B.V.Prescription requiredDosage form: PULMONARY INHALATION, 320 micrograms / 9 microgramsActive substance: formoterol and budesonideManufacturer: Teva Pharma B.V.Prescription requiredDosage form: PULMONARY INHALATION, 160 MICROGRAMS/4.5 MICROGRAMSActive substance: formoterol and budesonideManufacturer: Cipla Europe N.V.Prescription required
Online doctors for BUDESONIDE/FORMOTEROL CIPLA 320 mcg/9 mcg INHALATION POWDER (SINGLE DOSE)
Discuss questions about BUDESONIDE/FORMOTEROL CIPLA 320 mcg/9 mcg INHALATION POWDER (SINGLE DOSE), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















