
BUDESONIDE PULMICTAN INFANTILE 50 micrograms/INHALATION SUSPENSION FOR INHALATION IN A PRESSURIZED CONTAINER

How to use BUDESONIDE PULMICTAN INFANTILE 50 micrograms/INHALATION SUSPENSION FOR INHALATION IN A PRESSURIZED CONTAINER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Budesonida Pulmictan Infantil 50 micrograms/inhalation is and what it is used for
- What you need to know before taking Budesonida Pulmictan Infantil 50 micrograms/inhalation
- How to use Budesonida Pulmictan Infantil 50 micrograms/inhalation
- Possible side effects
- Storage of Budesonida Pulmictan Infantil 50 micrograms/inhalation
- Contents of the pack and further information
Introduction
Leaflet: Information for the user
BUDESONIDA PULMICTAN INFANTIL 50 micrograms/inhalation
suspension for inhalation in a pressurized container
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What Budesonida Pulmictan Infantil 50 micrograms/inhalation is and what it is used for.
- What you need to know before taking Budesonida Pulmictan Infantil 50 micrograms/inhalation.
- How to take Budesonida Pulmictan Infantil 50 micrograms/inhalation.
- Possible side effects.
- Storage of Budesonida Pulmictan Infantil 50 micrograms/inhalation.
- Contents of the pack and further information.
1. What Budesonida Pulmictan Infantil 50 micrograms/inhalation is and what it is used for
Budesonida Pulmictan Infantil 50 micrograms/inhalation contains the active substance budesonide. Budesonide belongs to a group of medicines called glucocorticoids, which are used to reduce inflammation.
Asthma is caused by inflammation of the airways. Budesonide reduces and prevents this inflammation.
Budesonida Pulmictan Infantil 50 micrograms/inhalation is used for the treatment of asthma. It should be used regularly as directed by your doctor.
When you inhale through the inhaler at the same time as you release a dose, the medicine will reach your lungs through the inhaled air.
2. What you need to know before taking Budesonida Pulmictan Infantil 50 micrograms/inhalation
Do not use Budesonida Pulmictan Infantil 50 micrograms/inhalation
-If you are allergic to budesonide or any of the components of Budesonida Pulmictan Infantil 50 micrograms/inhalation.
Tell your doctor so that your medicine can be changed to another one.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Budesonida Pulmictan Infantil 50 micrograms/inhalation.
Tell your doctor if:
- you are taking or have recently taken other medicines, including those purchased without a prescription.
- you have ever had an unusual reaction to Budesonida Pulmictan Infantil 50 micrograms/inhalation (budesonide) or any of its components, or to other medicines.
- you have or have had pulmonary tuberculosis.
- you have ever had liver problems.
- you have an untreated bacterial, viral, or fungal infection in your mouth, respiratory tract, or lungs.
- your doctor has prescribed Budesonida Pulmictan Infantil 50 micrograms/inhalation and you are still being treated with corticosteroid tablets, your doctor may gradually reduce the dose of these tablets (over a period of weeks or months) and may eventually stop the previous treatment.
In that case, some symptoms such as runny nose, hives, or muscle and joint pain may temporarily reappear. If any of these symptoms concern you, or if you experience others such as headache, fatigue, nausea, or vomiting, contact your doctor.
Contact your doctor if you experience blurred vision or other visual disturbances.
Rinse your mouth with water after each inhalation to avoid fungal infection in the mouth.
Budesonida Pulmictan Infantil 50 micrograms/inhalation has been prescribed for the maintenance treatment of asthma. However, it will not relieve an acute asthma attack once it has started.
Other medicines and Budesonida Pulmictan Infantil 50 micrograms/inhalation
Tell your doctor or pharmacist if you are using or have recently used any other medicine, including those purchased without a prescription.
Some medicines may increase the effects of Budesonida Pulmictan Infantil 50 micrograms/inhalation, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Tell your doctor if you are using:
- nasal medicines that contain corticosteroids
- corticosteroid tablets
- antifungal medicines containing ketoconazole and itraconazole
- cimetidine (a medicine for stomach acidity)
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
This medicine will only be used during pregnancy or breastfeeding when, in the doctor's opinion, the expected benefit to the mother is greater than any possible risk to the fetus.
Driving and using machines
Budesonida Pulmictan Infantil 50 micrograms/inhalation does not affect your ability to drive or use tools or machines.
Warning to athletes
Athletes are informed that this medicine contains a component that may produce a positive result in doping tests.
Budesonida Pulmictan Infantil 50 micrograms/inhalation contains ethanol
This medicine contains 0.33% ethanol (alcohol), which corresponds to 0.20 mg/dose.
3. How to use Budesonida Pulmictan Infantil 50 micrograms/inhalation
Follow these administration instructions for this medicine exactly as indicated by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Your doctor has indicated the duration of your treatment with Budesonida Pulmictan Infantil 50 micrograms/inhalation. Do not stop treatment before your doctor tells you to. Do not take more doses than your doctor has indicated. Consult your doctor if you have any questions about the treatment.
Method of use and administration route
Before starting treatment, you should know how the inhaler works. It is essential that you read the information included in the section "Instructions for the correct administration of the medicine" about the preparation, use, and cleaning of the inhaler and follow the instructions carefully.
Remember to always rinse your mouth with water after each inhalation.
The dose of Budesonida Pulmictan Infantil 50 micrograms/inhalation must be individualized. Your doctor will adjust the dose and prescribe the minimum that controls your asthma symptoms. Follow your doctor's instructions carefully.
Use in children and adolescents
Recommended dose in children 2-7 years: 4 inhalations (200 micrograms) - 8 inhalations (400 micrograms) per day, divided into 2-4 administrations.
Recommended dose in children from 7 years: 4 inhalations (200 micrograms) 16 inhalations (800 micrograms) per day, divided into 2-4 administrations.
The administration of Budesonida Pulmictan Infantil 50 micrograms/inhalation will be supervised by an adult to ensure that the dose is administered correctly and according to the doctor's instructions.
You may notice an improvement in symptoms even during the first day of treatment with Budesonida Pulmictan, although it may take 1 to 2 weeks to achieve a full effect. Therefore, it is essential that you do not stop using Budesonida Pulmictan even when you feel better.
In addition to the preventive Budesonida Pulmictan Infantil 50 micrograms/inhalation, you may also need a bronchodilator:
Budesonida will NOT stop an asthma attack once it has started. Therefore, you should always have a rapid-acting bronchodilator (a beta2 agonist) on hand in case you experience acute asthma symptoms.
If you use a calming inhaler (a beta2 agonist), you should inhale it before Budesonida Pulmictan Infantil 50 micrograms/inhalation (preventive).
Worsening of asthma symptoms during treatment:
Consult your doctor as soon as possible if:
- Your wheezing (wheezing) or chest pain worsens during treatment
- You need to use the calming inhaler more often than before
- Your calming inhaler does not relieve you as well as before
Your asthma may worsen, and you may need additional treatment.
Instructions for the correct administration of the medicine
Preparing the inhaler for use:
Remove the white cap. If it is a new inhaler or has not been used for several days, shake the aerosol and perform a puff to ensure the inhaler is working properly. If the inhaler is used regularly, proceed to the instructions for use.
Using the inhaler
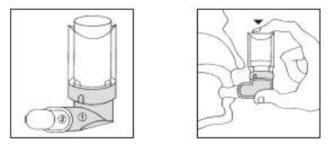
Inhalation with the aerosol only
- Check that the aerosol is properly assembled to the plastic mouthpiece (1). Shake the assembly and remove the white cap (2).
- Hold the canister in an inverted position between your thumb and index finger. Insert the mouthpiece into your mouth, pressing your lips around it.
- Perform a deep exhalation (expel air through your nose) and then inhale deeply through your mouth, pressing the canister between your fingers and causing a single discharge.
- Remove the device from your mouth and hold your breath for a few seconds. Exhale slowly and store the canister by putting the cap (2) back on.
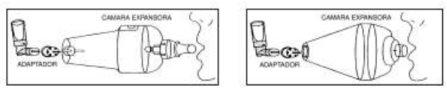
Inhalation with a spacer
- Check that the aerosol is properly assembled to the plastic applicator. Shake the assembly and remove the white cap.
- If necessary, attach the adapter to the mouthpiece of the applicator.
- Adjust the adapter, or the applicator itself, to the end of the spacer.
- Hold the spacer with the mouthpiece oriented towards your mouth. Press the canister between your thumb and index finger, causing the dose to be released inside the spacer.
- Exhale deeply and then insert the mouthpiece of the spacer into your mouth, pressing your lips around it.
- Inhale deeply through your mouth. Hold your breath for about ten seconds before exhaling through the spacer mouthpiece.
- Inhale again deeply to ensure the total inhalation of the administered dose. Hold your breath for a few seconds and exhale.
- Disconnect the aerosol from the spacer and adapter and store the canister by putting the white cap back on.
Cleaning
The mouthpiece-actuator should be cleaned regularly (at least once a week).
- Remove the actuator from the aerosol and rinse with plenty of water.
- Store with the cap on to protect it from dust and dirt.
If you use more Budesonida Pulmictan Infantil 50 micrograms/inhalation than you should
If you use a dose of Budesonida Pulmictan Infantil 50 micrograms/inhalation greater than you should in a single occasion, it is unlikely to cause harmful effects. If you have used too much Budesonida Pulmictan Infantil 50 micrograms/inhalation over a long period (months), it is possible that side effects may appear. In that case, consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone 91 562 04 20.
If you forget to use Budesonida Pulmictan Infantil 50 micrograms/inhalation
If you forget to use any of the doses of Budesonida Pulmictan Infantil 50 micrograms/inhalation, do not use a double dose to make up for the missed doses. Continue with the usual treatment as prescribed by your doctor.
If you stop treatment with Budesonida Pulmictan Infantil 50 micrograms/inhalation
Do not stop treatment with Budesonida Pulmictan Infantil 50 micrograms/inhalation without consulting your doctor. If you stop using the medicine abruptly, your asthma may worsen.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Common side effects:may affect up to 1 in 10 people
- Mild throat irritation
- Difficulty swallowing
- Cough
- Fungal infection of the mouth and throat (thrush)
To prevent the above-mentioned side effects, you can rinse your mouth and throat with water or brush your teeth after each dose. Do not swallow the water from the rinses, spit it out
Uncommon side effects:may affect up to 1 in 100 people
- Anxiety
- Depression
- Cataracts
- Blurred vision
- Muscle spasms and tremors
Rare side effects:may affect up to 1 in 1,000 people
- Low or high cortisol levels in the blood
- Underactive adrenal gland (gland near the kidneys)
- Skin rashes, itching, bruising
- Hoarseness
- Anxiety, nervousness
- Delayed growth and behavioral changes in children
Acute allergic reaction, rare:
If, shortly after taking a dose, you experience itching, rash, redness, swelling of the eyelids, lips, face, or throat, wheezing, low blood pressure, or collapse, act as follows:
- Stop taking Budesonida Pulmictan Infantil 50 micrograms/inhalation
- Seek medical advice immediately
Shortness of breath immediately after the dose:
Rarely, inhaled medicines can cause an increase in wheezing and shortness of breath (bronchospasm) immediately after the dose. If this happens:
- Stop taking Budesonida Pulmictan Infantil 50 micrograms/inhalation
- Take a rapid-acting bronchodilator
- Seek medical advice immediately
Very rare side effects:may affect up to 1 in 10,000 people
- Glaucoma
- Decreased bone density (weakening of the bones)
Side effects of unknown frequency(cannot be estimated from the available data):
- Sleep disturbances
- Aggressive reactions
- Increased motor activity (difficulty staying still)
- Irritability
These effects are more likely to occur in children.
If you were previously under treatment with corticosteroid tablets, switching to inhaled corticosteroid treatment may cause the appearance of some symptoms such as fatigue, abdominal pain, weakness, or vomiting. If you experience these symptoms, consult your doctor immediately.
If you think you may have any of these side effects, or if you are concerned about the possibility of having them, consult your doctor or pharmacist.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Budesonida Pulmictan Infantil 50 micrograms/inhalation
Keep this medicine out of the sight and reach of children.
The container that contains your medicine is pressurized. The valve should not be damaged, and the container should not be exposed to high temperatures or direct sunlight. Similarly, the container should not be punctured, broken, or burned, even if it is empty.
Do not refrigerate or freeze. Store below 30°C. Never expose the container to temperatures above 50°C.
Always keep the valve facing down.
Always put the protective cap on the mouthpiece after using the inhaler.
Do not use Budesonida Pulmictan after the expiration date that appears on the container. The expiration date is the last day of the month indicated.
Medicines should not be thrown away through wastewater or household waste. Deposit the containers and medicines you no longer need at the SIGRE collection point in your pharmacy. Ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Contents of the pack and further information
Composition of Budesonida Pulmictan Infantil 50 micrograms/inhalation:
- The active substance of Budesonida Pulmictan Infantil 50 micrograms/inhalation is budesonide. Each dose (1 inhalation) contains 50 micrograms of budesonide.
- The other components (excipients) are: oleic acid, ethanol, and 1,1,1,2-tetrafluoroethane (HFA 134a).
Appearance of the product and contents of the pack:
Budesonida Pulmictan Infantil 50 micrograms/inhalation is a suspension for inhalation through a pressurized container.
Each 10 ml container contains approximately 200 doses.
There are two concentrations of Budesonida Pulmictan in a pressurized container: Budesonida Pulmictan 200 micrograms/inhalation and Budesonida Pulmictan Infantil 50 micrograms/inhalation.
Marketing authorization holder and manufacturer:
Marketing authorization holder:
LABORATORIO REIG JOFRÉ, S.A.
Avda. Gran Capità, 10
08970 – Sant Joan Despí
Barcelona, Spain
Manufacturer:
LABORATORIO REIG JOFRÉ, S.A.
Avda. Gran Capità, 10
08970 – Sant Joan Despí
Barcelona, Spain
Or
GENETIC S.P.A.
Contrada Canfora, Fisciano, Salerno
Italy
Date of the last revision of this leaflet: February 2024
- Country of registration
- Average pharmacy price7.56 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUDESONIDE PULMICTAN INFANTILE 50 micrograms/INHALATION SUSPENSION FOR INHALATION IN A PRESSURIZED CONTAINERDosage form: PULMONARY INHALATION, 0.25 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.04000 gActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for BUDESONIDE PULMICTAN INFANTILE 50 micrograms/INHALATION SUSPENSION FOR INHALATION IN A PRESSURIZED CONTAINER
Discuss questions about BUDESONIDE PULMICTAN INFANTILE 50 micrograms/INHALATION SUSPENSION FOR INHALATION IN A PRESSURIZED CONTAINER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












