
BINOCRIT 4000 IU/0.4 ml INJECTABLE SOLUTION IN A PRE-FILLED SYRINGE

How to use BINOCRIT 4000 IU/0.4 ml INJECTABLE SOLUTION IN A PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Binocrit 1,000UI/0.5ml solution for injection in a pre-filled syringe
Binocrit 2,000UI/1ml solution for injection in a pre-filled syringe
Binocrit 3,000UI/0.3ml solution for injection in a pre-filled syringe
Binocrit 4,000UI/0.4ml solution for injection in a pre-filled syringe
Binocrit 5,000UI/0.5ml solution for injection in a pre-filled syringe
Binocrit 6,000UI/0.6ml solution for injection in a pre-filled syringe
Binocrit 7,000UI/0.7ml solution for injection in a pre-filled syringe
Binocrit 8,000UI/0.8ml solution for injection in a pre-filled syringe
Binocrit 9,000UI/0.9ml solution for injection in a pre-filled syringe
Binocrit 10,000UI/1ml solution for injection in a pre-filled syringe
Binocrit 20,000UI/0.5ml solution for injection in a pre-filled syringe
Binocrit 30,000UI/0.75ml solution for injection in a pre-filled syringe
Binocrit 40,000UI/1ml solution for injection in a pre-filled syringe
epoetin alfa
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Binocrit and what is it used for
- What you need to know before you use Binocrit
- How to use Binocrit
- Possible side effects
- Storing Binocrit
- Contents of the pack and other information
1. What is Binocrit and what is it used for
Binocrit contains the active substance epoetin alfa, a protein that stimulates the bone marrow to produce more red blood cells, which carry haemoglobin (a substance that carries oxygen). Epoetin alfa is a copy of the human protein erythropoietin and works in the same way.
Binocritis used to treat symptomatic anaemia caused by:
- Chronic kidney disease in adults and children on haemodialysis.
- Chronic kidney disease in adults on haemodialysis or peritoneal dialysis.
- Adults with severe anaemia who are not yet on dialysis.
If you have kidney disease, you may have a low number of red blood cells if your kidney does not produce enough erythropoietin (needed for red blood cell production). Binocrit is prescribed to stimulate the bone marrow to produce more red blood cells.
Binocrit is used to treat anaemia in adults receiving chemotherapyfor the treatment of solid tumours, malignant lymphoma, or multiple myeloma (bone marrow cancer) who may need a blood transfusion. Binocrit may reduce the need for a blood transfusion in these patients.
Binocrit is used in adults with moderate anaemia who are donating their own blood before surgery, so that it can be given back to them during or after surgery. Since Binocrit stimulates red blood cell production, doctors can take more blood from these patients.
Binocrit is used in adults with moderate anaemia who are about to have major orthopaedic surgery(e.g. hip or knee replacement), to reduce the possible need for blood transfusions.
Binocrit is used to treat anaemia in adults with a bone marrow disorder that leads to a severe disruption of blood cell formation (myelodysplastic syndromes).Binocrit may reduce the need for a blood transfusion.
2. What you need to know before you use Binocrit
Do not use Binocrit
- If you are allergicto epoetin alfa or any of the other ingredients of this medicine (listed in section 6).
- If you have been diagnosed with pure red cell aplasia(the bone marrow cannot produce enough red blood cells) after treatment with any product that stimulates red blood cell production (including Binocrit). See section 4.
- If you have uncontrolled high blood pressure.
- To stimulate red blood cell production (so that your doctors can take more blood from you), if you cannot receive transfusions of your own bloodduring or after surgery.
- If you are about to have major elective orthopaedic surgery(e.g. hip or knee replacement) and you:
- have severe heart disease,
- have severe disorders of the veins and arteries,
- have recently had a heart attack or stroke,
- cannot take medicines to thin your blood.
Binocrit may not be suitable for you. Talk to your doctor. Some people need medicines to reduce the risk of blood clots during treatment with Binocrit. If you cannot take medicines to prevent blood clots, you should not take Binocrit.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Binocrit.
Binocrit and other products that stimulate red blood cell production may increase the risk of blood clots in all patients.This risk may be greater if you have other risk factors for blood clots (e.g. if you have had a blood clot in the past or have a high body mass index, diabetes, heart disease, or are bedridden for a long time due to surgery or illness). Tell your doctor about any of these. Your doctor will help you decide if Binocrit is suitable for you.
It is important that you tell your doctorif you identify with any of the following situations.You may still be able to use Binocrit, but discuss it with your doctor first.
If you know that you haveor have had:
- high blood pressure;
- seizures or convulsions;
- a liver disease;
- anaemia of other causes;
- porphyria (a rare blood disorder).
If you are a patient with chronic kidney disease,and especially if you do not respond well to Binocrit, your doctor will check your Binocrit dose, because repeatedly increasing the dose of Binocrit if you do not respond to treatment may increase the risk of heart or blood vessel problems and may increase the risk of heart attack, stroke, and death.
If you are a cancer patient,you should know that products that stimulate red blood cell production (like Binocrit) may act as a growth factor and therefore, in theory, affect the progression of cancer.
Depending on your individual situation, a blood transfusion may be preferable. Discuss this with your doctor.
If you are a cancer patient,you should know that the use of Binocrit may be associated with lower survival and higher mortality rates in patients with head and neck cancer and metastatic breast cancer receiving chemotherapy.
Severe skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been observed with the administration of epoetins.
SJS/TEN can start with a red, flat rash that looks like a target, often with central blisters, on the trunk. You may also have ulcers in your mouth, throat, nose, genitals, and eyes (redness and swelling). These severe skin rashes can progress to widespread skin peeling and potentially life-threatening complications.
If you develop a severe skin rash or any of these other skin symptoms, stop taking Binocrit and contact your doctor or seek medical attention immediately.
Be careful with other products that stimulate red blood cell production:
Binocrit is a product belonging to a group of products that stimulate red blood cell production, like erythropoietin, which is a human protein. Your healthcare professional will always write down the exact product you are using. If, during your treatment, you are given a product belonging to this group that is different from Binocrit, talk to your doctor or pharmacist before using it.
Other medicines and Binocrit
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
If you are a patient with hepatitis C and are receiving interferon and ribavirin
Tell your doctor, as the combination of epoetin alfa with interferon and ribavirin has led to a loss of effect and the occurrence of a disease called pure red cell aplasia (PRCA), a severe form of anaemia, in rare cases. Binocrit is not approved for the treatment of anaemia associated with hepatitis C.
If you are taking a medicine called cyclosporin(which is used, for example, after a kidney transplant), your doctor may ask for blood tests to check the level of cyclosporin while you are being treated with Binocrit.
Iron supplements and other blood stimulantsmay increase the effectiveness of Binocrit. Your doctor will decide whether it is suitable for you to take them.
If you visit a hospital, clinic, or general practitioner, tell them that you are being treated with Binocrit, as it may affect other treatments or test results.
Pregnancy, breast-feeding, and fertility
It is important that you tell your doctorif you identify with any of the following situations.You may still be able to use Binocrit, but discuss it with your doctor first:
- if you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There are no data on the effects of Binocrit on fertility
Binocrit contains sodium:
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How to use Binocrit
Follow your doctor's instructions for administering this medication exactly.If you are in doubt, consult your doctor again.
Your doctor has performed blood testsand has decided that you need Binocrit.
Binocrit can be administered by injection:
- Eitherin a vein or through a tube inserted into a vein (intravenously).
- Orunder the skin (subcutaneously).
Your doctor will decide how Binocrit will be injected. The injections will usually be performed by a doctor, nurse, or other healthcare professional. Later, and depending on the reason you need treatment with Binocrit, some people may learn to self-inject the medication under the skin: see Instructions on how to inject Binocritat the end of the prospectus.
Binocrit should not be used:
- after the expiration date on the label and carton;
- if you know or believe it may have been accidentally frozen, or
- if there has been a refrigerator failure.
The dose of Binocrit you receive is based on your body weight in kilograms. The cause of your anemia is also a factor your doctor will consider when deciding the correct dose.
Your doctor will periodically check your blood pressurewhile you are being treated with Binocrit.
People with kidney disease
- Your doctor will keep your hemoglobin levels between 10 and 12 g/dl, as high hemoglobin levels can increase the risk of blood clots and death. In children, the hemoglobin level should be kept between 9.5 and 11 g/dl.
- The usual initial doseof Binocrit for adults and children is 50 international units (IU) per kilogram (kg) of body weight, administered three times a week. In patients on peritoneal dialysis, Binocrit can be administered twice a week.
- In both adults and children, Binocrit is administered by injection, either in a vein (intravenously) or through a tube inserted into a vein. When venous access cannot be easily obtained, your doctor may decide that Binocrit should be injected under the skin (subcutaneously). This includes patients on dialysis and those who are not yet on dialysis.
- Your doctor will request periodic blood tests to see how your anemia is responding, usually no more than every four weeks, and may adjust the dose. An increase in hemoglobin of more than 2 g/dl during a four-week period should be avoided.
- Once the anemia has been corrected, your doctor will continue to perform periodic blood tests. They may adjust the dose and frequency of Binocrit administration to maintain your response to treatment. Your doctor will use the minimum effective dose to control the symptoms of anemia.
- If you do not respond well to Binocrit, your doctor will check your dose and inform you if you need to modify the dose of Binocrit.
- If you receive a wider dosing interval (more than once a week) of Binocrit, you may not maintain an adequate hemoglobin concentration and may require an increase in the dose of Binocrit or the frequency of administration.
- You may be given iron supplements before and during treatment with Binocrit to increase its effectiveness.
- If you are on dialysis at the start of therapy with Binocrit, it may be necessary to adjust your dialysis schedule. Your doctor will decide if this is necessary.
Adults undergoing chemotherapy
- Your doctor may start treatment with Binocrit if your hemoglobin level is 10 g/dl or lower.
- Your doctor will keep your hemoglobin levels between 10 and 12 g/dl, as high hemoglobin levels can increase the risk of blood clots and death.
- The initial dose is 150 IU per kilogram of body weight three times a week, or450 IU per kilogram of body weight once a week.
- Binocrit is administered by injection under the skin.
- Your doctor will request blood tests and may adjust the dose, depending on how your anemia responds to treatment with Binocrit.
- You may be given iron supplements before and during treatment with Binocrit to increase its effectiveness.
- You will usually continue treatment with Binocrit for a month after the end of chemotherapy.
Adults who donate their own blood
- The usual doseis 600 IU per kilogram of body weight twice a week.
- Binocrit is administered by intravenous injection immediately after blood donation during the three weeks prior to surgery.
- You may be given iron supplements before and during treatment with Binocrit to increase its effectiveness.
Adults scheduled for major orthopedic surgery
- The recommended doseis 600 IU per kilogram of body weight once a week.
- Binocrit is administered by injection under the skin every week for the three weeks prior to surgery and on the day of surgery.
- If there is a medical need to reduce the time before surgery, you will be given a daily dose of 300 IU/kg for a maximum of ten days before surgery, on the day of surgery, and for the four days immediately after.
- Treatment will be discontinued if blood tests show that your hemoglobin is too high before surgery.
- You may be given iron supplements before and during treatment with Binocrit to increase its effectiveness.
Adults with myelodysplastic syndrome
- Your doctor may start treatment with Binocrit if your hemoglobin level is 10 g/dl or lower. The goal of treatment is to keep your hemoglobin level between 10 and 12 g/dl, as higher hemoglobin levels can increase the risk of blood clots and death.
- Binocrit is administered by injection under the skin.
- The initial dose is 450 IU per kilogram of body weight once a week.
- Your doctor will request blood tests and may adjust the dose, depending on how your anemia responds to treatment with Binocrit.
Instructions on how to inject Binocrit
When starting treatment, medical or nursing staff usually inject Binocrit. Later, your doctor may suggest that you or your caregiver learn to inject Binocrit under the skin (subcutaneously) by yourself.
- Do not attempt to self-inject unless your doctor or nurse has taught you how to do so.
- Follow your doctor's or nurse's instructions for administering Binocrit exactly.
- Make sure to inject only the amount of liquid indicated by your doctor or nurse.
- Only useBinocritif it has been stored correctly; see section5,Storage of Binocrit.
- Before use, let the Binocrit syringe stand at room temperature until it reaches room temperature. This usually takes between 15 and 30minutes. Use the syringe within 3days of removing it from the refrigerator.
Only withdraw one dose of Binocrit from each syringe.
If Binocrit is injected under the skin (subcutaneously), the amount injected is usually no more than one milliliter (1 ml) per injection.
Binocrit is administered alone and not mixed with other injectable liquids.
Do not shake Binocrit syringes.Prolonged and vigorous shaking can damage the product. If the product has been shaken vigorously, do not use it.
At the end of this prospectus, you can find the Instructions on how to inject Binocrit.
If you use more Binocrit than you should
Inform your doctor or nurse immediately if you think you have injected too much Binocrit. It is unlikely that adverse effects will occur as a result of an overdose of Binocrit.
If you forget to use Binocrit
Take the next injection as soon as you remember. If it is less than a day until the next injection, skip the missed dose and continue with your regular schedule. Do not take a double dose to make up for missed doses.
If you have any other questions about using this medication, ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse immediatelyif you notice any of the effects on this list.
Severe skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been observed with the administration of epoetins. These reactions can appear as red, circular patches, often with central blisters, on the trunk, skin peeling, and ulcers in the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms. Stop using Binocrit if you experience these symptoms and contact your doctor or seek medical attention immediately. See also section 2.
Very common side effects
May affect more than 1 in 10 people.
- Diarrhea.
- Feeling of nausea.
- Vomiting.
- Fever.
- Congestion of the respiratory tract, such as a stuffy nose and sore throat, which have been reported in patients with kidney disease who are not yet on dialysis.
Common side effects
May affect up to 1 in 10 people.
- Increased blood pressure. Headaches, especially sudden, severe, and migrainous, feeling of confusion or seizures, can be signs of a sudden increase in blood pressure. This requires urgent treatment. Increased blood pressure may require treatment with other medications (or adjustment of any medication you are already taking for high blood pressure).
- Blood clots(including deep vein thrombosis and embolism) that may require urgent treatment. You may experience chest pain, difficulty breathing, and painful swelling and redness, usually of one leg, as symptoms.
- Cough.
- Skin rash, which can occur as a result of an allergic reaction.
- Bone or muscle pain.
- Flu-like symptoms, such as headache, joint pain, feeling of weakness, chills, fatigue, and dizziness. These may be more frequent at the start of treatment. If these symptoms occur during injection into a vein, slower administration of the injection may help avoid them in the future.
- Redness, itching, and pain at the injection site.
- Swelling of the ankles, feet, or fingers.
Uncommon side effects
May affect up to 1 in 100 people.
- High levels of potassium in the bloodthat can cause an abnormal heart rhythm (this is a very common side effect in patients on hemodialysis).
- Seizures.
- Congestion of the nose or respiratory tract.
Rare side effects
May affect up to 1 in 1,000 people.
- Symptoms of pure red cell aplasia (PRCA)
PRCA means that the bone marrow does not produce enough red blood cells. PRCA causes sudden and severe anemia. The symptoms are:
- Unusual fatigue.
- Feeling of dizziness.
- Difficulty breathing.
PRCA has been reported in very rare cases, mainly in patients with kidney disease after months or years of treatment with epoetin alfa and other products that stimulate red blood cell production.
- There may be an increase in the number of small blood cells (platelets) that normally participate in blood clot formation, especially at the start of treatment. Your doctor will check this.
If you are on hemodialysis:
- Blood clots(thrombosis) may form in the arteriovenous shunt of your dialysis. This is more likely if you have low blood pressure or if your fistula has complications.
- Blood clotsmay also form in your hemodialysis system. Your doctor may decide to increase the dose of heparin during dialysis.
Tell your doctor or nurse immediatelyif you notice any of these effects or if you notice any other effect while receiving treatment with Binocrit.
If you think any of the side effects you are experiencing are serious, or if you notice any side effects not mentioned in this prospectus, tell your doctor, nurse, or pharmacist.
Reporting side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Binocrit
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiration date stated on the label and carton after EXP. The expiration date is the last day of the month indicated.
- Store and transport refrigerated (between 2°C and 8°C).
- You can remove Binocrit from the refrigerator and store it at room temperature (up to 25°C) for a period not exceeding three days. Once a syringe has been removed from the refrigerator and has reached room temperature (up to 25°C), it must be used within three days or discarded.
- Do not freeze or shake.
- Keep in the original packaging to protect from light.
Do not use this medicine if you notice
- that it may have been accidentally frozen or
- a refrigerator failure has occurred,
- the liquid is colored or you notice particles floating in it,
- the seal is broken.
Medicines should not be disposed of via wastewater.Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container contents and additional information
Composition of Binocrit
- The active substance is: epoetin alfa (to know the quantity, see the table below).
- The other components are: sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, sodium chloride, glycine, polysorbate 80, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment), and water for injectable solutions.
Appearance of the product and container contents
Binocrit is presented as a clear and colorless injectable solution, for injection in a pre-filled syringe. The syringes are sealed in a blister pack.
Presentation | Corresponding presentations in quantity/volume per dose presentation | Quantity of epoetin alfa |
Pre-filled syringes* | 2,000 IU/ml: 1,000 IU/0.5 ml 2,000 IU/1 ml 10,000 IU/ml: 3,000 IU/0.3 ml 4,000 IU/0.4 ml 5,000 IU/0.5 ml 6,000 IU/0.6 ml 7,000 IU/0.7 ml 8,000 IU/0.8 ml 9,000 IU/0.9 ml 10,000 IU/1 ml 40,000 IU/ml: 20,000 IU/0.5 ml 30,000 IU/0.75 ml 40,000 IU/1 ml | 8.4 micrograms 16.8 micrograms 25.2 micrograms 33.6 micrograms 42.0 micrograms 50.4 micrograms 58.8 micrograms 67.2 micrograms 75.6 micrograms 84.0 micrograms 168.0 micrograms 252.0 micrograms 336.0 micrograms |
*Container size of 1, 4, or 6 pre-filled syringe(s) with or without a needle safety guard.
Only some pack sizes may be marketed.
Marketing authorization holder
Sandoz GmbH
Biochemiestr. 10
6250 Kundl
Austria
Manufacturer
Sandoz GmbH
Biochemiestr. 10
6336 Langkampfen
Austria
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Sandoz nv/sa Tél/Tel: +32 2 722 97 97 | Lietuva Sandoz Pharmaceuticals d.d filialas Tel: +370 5 2636 037 |
България Сандоз България ЕООД Тел.: +359 2 970 47 47 | Luxembourg/Luxemburg Sandoz nv/sa Tél/Tel.: +32 2 722 97 97 |
Česká republika Sandoz s.r.o. Tel: +420 225 775 111 | Magyarország Sandoz Hungária Kft. Tel.: +36 1 430 2890 |
Danmark/Norge/Ísland/Sverige Sandoz A/S Tlf: +45 63 95 10 00 | Malta Sandoz Pharmaceuticals d.d. Tel: +35699644126 |
Deutschland Hexal AG Tel: +49 8024 908 0 | Nederland Sandoz B.V. Tel: +31 36 52 41 600 |
Eesti Sandoz d.d. Eesti filiaal Tel: +372 665 2400 | Österreich Sandoz GmbH Tel: +43 5338 2000 |
Ελλάδα ΣΑΝΔΟΖ ΕΛΛΑΣ ΜΟΝΟΠΡΟΣΩΠΗ Α.Ε. Τηλ: +30 216 600 5000 | Polska Sandoz Polska Sp. z o.o. Tel.: +48 22 209 70 00 |
España Sandoz Farmacéutica, S.A. Tel: +34 900 456 856 | Portugal Sandoz Farmacêutica Lda. Tel: +351 21 000 86 00 |
France Sandoz SAS Tél: +33 1 49 64 48 00 | România Sandoz Pharmaceuticals SRL Tel: +40 21 407 51 60 |
Hrvatska Sandoz d.o.o. Tel: +385 1 23 53 111 | Slovenija Sandoz farmacevtska družba d.d. Tel: +386 1 580 29 02 |
Ireland Rowex Ltd. Tel: + 353 27 50077 | Slovenská republika Sandoz d.d. - organizačná zložka Tel: +421 2 50 70 6111 |
Italia Sandoz S.p.A. Tel: +39 02 96541 | Suomi/Finland Sandoz A/S Puh/Tel: +358 10 6133 400 |
Κύπρος Sandoz Pharmaceuticals d.d. Τηλ: +357 22 69 0690 | United Kingdom (Northern Ireland) Sandoz GmbH Tel: +43 5338 2000 |
Latvija Sandoz d.d. Latvia filiale Tel: +371 67 892 006 |
Date of last revision ofthis leaflet: {MM/YYYY}.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
------------------------------------------------------------------------------------------------------------------
Self-injection instructions (for patients withsymptomatic anemia caused by a kidney disease, for adult patients receiving chemotherapy, adult patients scheduled for major surgery, or adult patients with myelodysplastic syndromes)
This section contains information on how to administer Binocrit to yourself. It is important that you do not attempt to inject yourself without your doctor or nurse having first explained how to do it.Binocrit may be supplied with or without a needle safety guard, which your doctor or nurse will show you how to use. If you are unsure about injecting yourself or have any questions, consult your doctor or nurse.
WARNING: Do not use if the syringe has been dropped onto a hard surface or has been dropped after removing the needle cap. Do not use the pre-filled Binocrit syringe if it is broken. Return the pre-filled syringe and its packaging to the pharmacy.
- Wash your hands.
- Remove a syringe from the packaging and remove the needle cap. The syringes are graduated with markings to allow for partial use if necessary. Each marking corresponds to a volume of 0.1 ml. If partial use of the syringe is required, discard the unwanted solution before injection.
- Clean the skin at the injection site with an alcohol swab.
- Form a fold in the skin by pinching the skin between your thumb and index finger.
- Insert the needle into the skin fold at a 45-degree angle, with a quick and firm action. Inject the Binocrit solution as your doctor has shown you. Consult your doctor or pharmacist if you have any doubts.
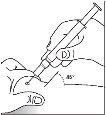 Pre-filled syringe without needle safety guard
Pre-filled syringe without needle safety guard
- While keeping the skin pinched, press the plunger slowly and evenly.
- Once the liquid has been injected, remove the needle and release the skin. Apply pressure to the injection site with a dry, sterile swab.
- Discard any unused medication and all materials that have come into contact with it. Use each syringe only for one injection.
Pre-filled syringe with needle safety guard
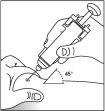 While keeping the skin pinched, press the plunger slowly and evenly until the entire dose has been administered and the plunger can no longer be advanced. Do not release pressure on the plunger!
While keeping the skin pinched, press the plunger slowly and evenly until the entire dose has been administered and the plunger can no longer be advanced. Do not release pressure on the plunger!- Once the liquid has been injected, remove the needle while maintaining pressure on the plunger and release the skin. Apply pressure to the injection site with a dry, sterile swab.
- Release the plunger. The needle safety guard will move quickly to cover the needle.
- Discard any unused medication and all materials that have come into contact with it. Use each syringe only for one injection.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BINOCRIT 4000 IU/0.4 ml INJECTABLE SOLUTION IN A PRE-FILLED SYRINGEDosage form: INJECTABLE, 20000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 20,000 IUActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40,000 IU/ml of epoetin alfaActive substance: erythropoietinManufacturer: Sandoz GmbhPrescription required
Online doctors for BINOCRIT 4000 IU/0.4 ml INJECTABLE SOLUTION IN A PRE-FILLED SYRINGE
Discuss questions about BINOCRIT 4000 IU/0.4 ml INJECTABLE SOLUTION IN A PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















