
ATROVENT NASAL 0.30 mg/ml NASAL SPRAY SOLUTION

How to use ATROVENT NASAL 0.30 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Atrovent Nasal 0.30 mg/ml nasal spray solution
Ipratropium bromide
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the pack:
- What Atrovent Nasal is and what it is used for
- Before using Atrovent Nasal
- How to use Atrovent Nasal
- Possible side effects
- Storing Atrovent Nasal
- Further information
1. What Atrovent Nasal is and what it is used for
Atrovent Nasal belongs to a group of medicines called anticholinergic bronchodilators, which work by relaxing the muscle in the airways, making it easier to breathe.
Rhinitis is an inflammation of the mucous membranes of the nasal passages. Atrovent Nasal is a medicine used to relieve the symptoms of runny nose (rhinorrhea) caused by allergic and non-allergic rhinitis.
2. Before using Atrovent Nasal
Do not use Atrovent Nasal
- If you are allergic (hypersensitive) to ipratropium bromide or similar substances such as atropine or its derivatives, or to any of the other ingredients of Atrovent Nasal.
Take special care with Atrovent Nasal
- If you have a predisposition to narrow-angle glaucoma (increased pressure inside the eye), prostate enlargement, or obstruction of the neck of the bladder (if the bladder outlet is obstructed).
- If you have cystic fibrosis (a disease that affects the mucous and sweat glands, affecting several organs), you may be more prone to gastrointestinal motility disorders.
After using Atrovent Nasal, immediate hypersensitivity reactions (rapid allergic reactions) such as hives, angioedema (sudden swelling of the skin or mucous membranes), buccopharyngeal edema (swelling in the mouth and pharynx), and anaphylaxis (generalized allergic reaction) may occur.
Isolated cases of eye complications have been reported, such as mydriasis (pupil dilation), narrow-angle glaucoma (increased pressure inside the eye), eye pain from spraying ipratropium bromide aerosol into the eyes alone or in combination with a beta-2 adrenergic agonist (medicines that dilate bronchial muscles, such as salbutamol). Therefore, it is essential to strictly follow the doctor's instructions for the correct administration of Atrovent Nasal.
Eye pain or discomfort, blurred vision, halos (diffuse lights), or colored images, along with redness of the eye due to conjunctival congestion and corneal edema (loss of corneal transparency), may be signs of acute narrow-angle glaucoma (increased pressure inside the eye). If any combination of these symptoms is observed, the doctor should be notified immediately.
Using other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription.
It has not been reported that the use of Atrovent Nasal with other medications commonly prescribed for perennial rhinitis (rhinitis that occurs throughout the year), such as antihistamines, decongestants, or nasal steroids, increases the occurrence of side effects. Although Atrovent Nasal passes into the bloodstream only in minimal amounts, there is a possibility that its effect may be enhanced if administered with other anticholinergic medications, including ipratropium bromide inhalation aerosols.
Pregnancy
Consult your doctor or pharmacist before using any medicine.
Although preclinical studies have not shown any risk, the safety of Atrovent Nasal during pregnancy has not been established. In case of suspected or confirmed pregnancy, the benefits of treatment should be weighed against the potential risks to the fetus.
Breast-feeding
Consult your doctor or pharmacist before using any medicine.
It is unknown whether ipratropium bromide can pass into breast milk. However, it is unlikely that ipratropium bromide will pass to the nursing infant in significant amounts, especially when administered nasally. Nevertheless, since many medications can pass into breast milk, it should be administered with caution to nursing mothers.
Driving and using machines
There are no studies on the effects on the ability to drive and use machines. However, it is warned that side effects such as dizziness, eye difficulties in focusing, pupil dilation, and blurred vision may occur during treatment with Atrovent Nasal. Therefore, caution is recommended when driving and using machines. If patients experience these effects, they should avoid performing potentially hazardous activities such as driving or using machines.
Atrovent Nasal contains benzalkonium chloride
This medicine contains 0.25 mg of benzalkonium chloride per ml.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for extended treatment periods.
3. How to use Atrovent Nasal
Follow the instructions for using Atrovent Nasal exactly as indicated by your doctor. Consult your doctor or pharmacist if you have any doubts.
Your doctor will determine the duration of treatment.
If you think the effect of Atrovent Nasal is too strong or too weak, tell your doctor or pharmacist.
The administration of Atrovent Nasal should be adapted to the individual needs of each patient; patients should be under medical supervision during treatment.
The normal dose is:
Adults and adolescents over 12 years:2 sprays in each nostril, 2-3 times a day.
Children from 6 to 12 years:2 sprays in each nostril, 2 times a day.
However, the optimal dose may vary according to the individual response of each patient and should be established by the doctor.
Method of administration:
- Remove the protective cap.
- Before using the spray for the first time, press the spray several times (approximately 7 times) until the first spray is released (see figure 1). To activate the pump, hold the bottle with your thumb, index, and middle fingers.
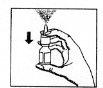 Make sure the bottle is pointing upwards and is away from the eyes. Press the bottle firmly and quickly with your thumb (figure 1). The pump will now be ready for use.
Make sure the bottle is pointing upwards and is away from the eyes. Press the bottle firmly and quickly with your thumb (figure 1). The pump will now be ready for use.
(figure 1)
If the pump has not been used for more than 24 hours, it should be reactivated with 1 or 2 sprays.
- Before using the nasal spray, blow your nose to clean the nasal passages.
- Close one nostril with your finger and tilt your head slightly forward. With the bottle held as shown in figure 1, insert the nasal applicator into the other nostril (see figure 2). Direct the applicator towards the back and outer part of the nose.

(figure 2)
- Activate the pump once, pressing firmly and quickly upwards with your thumb. After each spray, inhale (breathe in) deeply and exhale (breathe out) through the mouth.
- After spraying and removing the applicator, tilt your head back for a few seconds to allow the spray to spread over the back of the nose.
- Apply another spray in the same nostril, following the same procedure.
- Apply two sprays in the other nostril, following the same instructions.
- Replace the protective cap after use.
If Atrovent Nasal is accidentally sprayed into the eyes, rinse them immediately with cold water.
If the nasal applicator becomes blocked, remove the protective cap. Place the nasal applicator under a hot water tap for about one minute. Dry the nasal applicator, activate the pump (operation 2), and replace the protective cap.
If you use more Atrovent Nasal than you should
No specific symptoms of overdose have been reported. Due to the wide safety margin and the fact that Atrovent Nasal is used topically, no serious anticholinergic symptoms are expected. Minor anticholinergic symptoms such as dry mouth, visual accommodation disorders, and increased heart rate may occur.
If you have used more Atrovent Nasal than you should, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
4. Possible side effects
Like all medicines, Atrovent Nasal can cause side effects, although not everybody gets them.
Common side effects (occurring in at least 1 in 100 patients) are headache; throat irritation, nasal dryness, nasal bleeding, and nasal discomfort.
Uncommon side effects (occurring in at least 1 in 1,000 patients) are anaphylactic reactions (severe allergic reactions), hypersensitivity, dizziness, halos (diffuse lights) or colored images associated with eye redness (glaucoma), increased intraocular pressure, eye pain, pupil dilation, visual accommodation disorder (difficulty focusing), blurred vision, halos (diffuse lights), eye redness, corneal edema (corneal swelling), supraventricular tachycardia, atrial fibrillation, increased heart rate, dry mouth and throat, bronchospasm (chest tightness, wheezing, or shortness of breath), laryngospasm (contraction of the larynx that causes difficulty breathing), pharyngeal edema (throat swelling), oral edema (mouth swelling), stomatitis (mouth inflammation), skin rash, angioedema (swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing or breathing), urinary retention, gastrointestinal motility disorders, and nausea.
Rare side effects (occurring in at least 1 in 10,000 patients) are palpitations, pruritus (itching), and urticaria.
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Atrovent Nasal
Keep out of the reach and sight of children.
Do not use Atrovent Nasal after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Do not use after 12 months of opening the packaging for the first time.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Further information
Composition of Atrovent Nasal
- The active ingredient is ipratropium bromide. Each ml of solution contains 0.31 mg of ipratropium bromide monohydrate (equivalent to 0.30 mg of anhydrous ipratropium bromide). One spray delivers 21.7 micrograms of ipratropium bromide monohydrate, equivalent to 21 micrograms of anhydrous ipratropium bromide.
- The other ingredients are: sodium chloride, benzalkonium chloride, disodium edetate, hydrochloric acid, and purified water.
Appearance and packaging
15 ml (180 sprays) of nasal spray solution.
Marketing authorization holder
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona), Spain
Manufacturer
Istituto De Angeli S.r.l.
Localita i Prulli
50066 Reggello (Florence)
or
BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG
Birkendorfer Strasse, 65
D-88397 Biberach am der Riss
Germany
This leaflet was approved in July 2013
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price6.15 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATROVENT NASAL 0.30 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 12500 IU/gActive substance: retinolManufacturer: Laboratorio Stada S.L.Prescription not requiredDosage form: NASAL PRODUCT, 850 mg sodium chloride / 100 mlActive substance: variousManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: NASAL PRODUCT, 0.1 g azelastine hydrochloride/100 mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription required
Online doctors for ATROVENT NASAL 0.30 mg/ml NASAL SPRAY SOLUTION
Discuss questions about ATROVENT NASAL 0.30 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











