
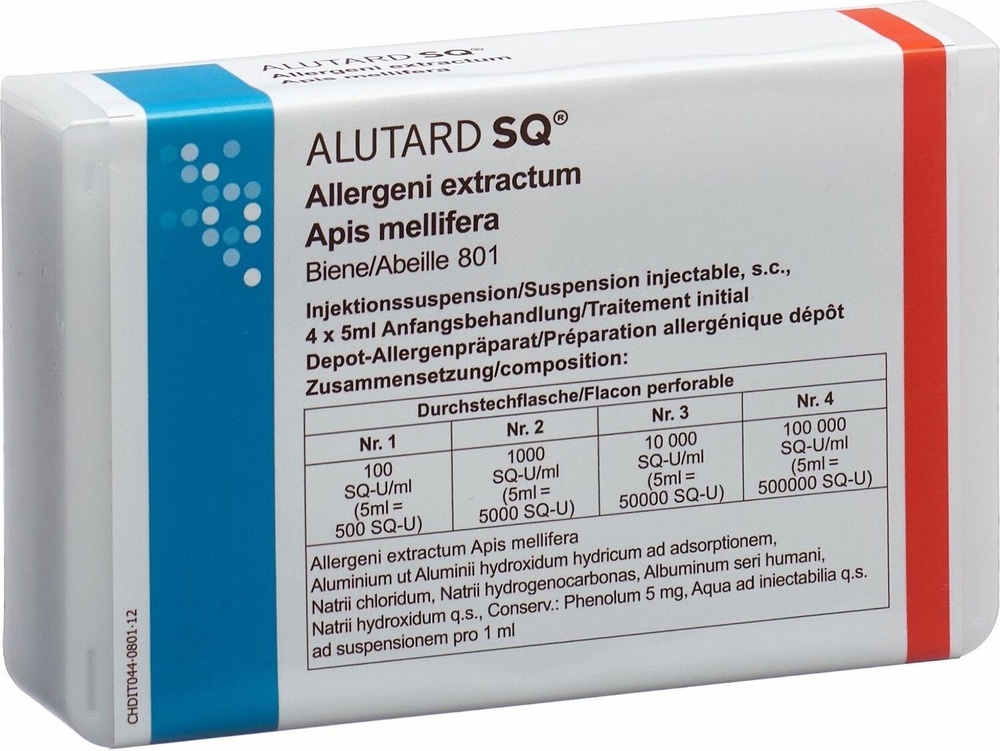
ALUTARD SQ APIS MELLIFERA Starter Pack (100 SQ-U/mL, 1,000 SQ-U/mL, 10,000 SQ-U/mL and 100,000 SQ-U/mL) Injectable Suspension

How to use ALUTARD SQ APIS MELLIFERA Starter Pack (100 SQ-U/mL, 1,000 SQ-U/mL, 10,000 SQ-U/mL and 100,000 SQ-U/mL) Injectable Suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Alutard SQ Apis mellifera starter kit (100 SQ-U/ml, 1,000 SQ-U/ml, 10,000 SQ-U/ml, and
100,000 SQ-U/ml), injectable suspension
Alutard SQ Apis mellifera 100,000 SQ-U/ml injectable suspension
Bee venom allergen (Apis mellifera)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Alutard SQ Apis mellifera is and what it is used for
- What you need to know before you use Alutard SQ Apis mellifera
- How to use Alutard SQ Apis mellifera
- Possible side effects
- Storage of Alutard SQ Apis mellifera
- Contents of the pack and other information
1. What Alutard SQ Apis mellifera is and what it is used for
Alutard SQ Apis mellifera contains the allergen (the substance that causes the allergic reaction) of bee venom. It is used as a preventive treatment for bee sting allergy.
This treatment is used in patients with a history of severe allergic reactions to bee stings. The goal of the treatment is to combat the underlying cause of the allergy. It works by gradually increasing immunological tolerance to bee venom.
2. What you need to know before you use Alutard SQ Apis mellifera
Do not use Alutard SQ Apis mellifera
- if you are allergic to any of the other ingredients of this medicine (listed in section 6).
- if you have a disease that affects the immune system.
- if you have recently had an asthma attack and/or your asthma symptoms have worsened, for example, your symptoms have increased during the day, you have had nighttime awakenings, your need for medication has increased, and/or you have had limitations in your activity.
- if you have severe heart or vascular disease.
Warnings and precautions
Talk to your doctor before starting to use Alutard SQ Apis mellifera if:
- you have experienced any side effects after the last administration of Alutard SQ Apis mellifera
- you have chronic heart disease
- you know that your kidney function is reduced, as there may be a risk of aluminum accumulation in your body
- you have an autoimmune disease
- you have cancer
- you have a fever or show any other sign of infection
- in the last 3 or 4 days you have had allergic symptoms such as allergic rhinitis
- you have eczema that has worsened
- you know that you have a high level of tryptase protein in your blood
- you know that you have mastocytosis or any other disease that produces an increase in the number of mast cells in your body
- you have asthma
If you have any of the above, it is essential that you inform your doctor about it, to reduce the risk of allergic reactions related to treatment with Alutard SQ Apis mellifera (see section 4 "Possible side effects").
Children and adolescents
Children from 5 years old: The information on the effect of the treatment in children is limited. Safety data have not shown a higher risk in children than in adults. It is recommended that the doctor assess the risks and benefits in each child.
Children under 5 years old: The doctor should carefully assess the risks and benefits of treatment in each child.
Using Alutard SQ Apis mellifera with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor or nurse especially if
- you are taking any other medicine to treat your allergy, such as antihistamines or corticosteroids, as they may increase your tolerance to this treatment. The doctor may need to adjust the dose
- you are taking medicines that contain large amounts of aluminum, such as some antacids (used for heartburn). Since Alutard SQ Apis mellifera also contains aluminum, there may be a risk of aluminum accumulation in your body
- you have been vaccinated recently, for example against tetanus. At least one week should pass between the injection of Alutard SQ Apis mellifera and that of the other vaccine
- you are taking beta-blockers or ACE inhibitors to treat high blood pressure or heart disease, tricyclic antidepressants or monoamine oxidase inhibitors (MAOIs) for depression, or COMT inhibitors for Parkinson's disease. These medicines may increase the risk of and/or influence the treatment of any allergic reaction produced when using Alutard SQ Apis mellifera
Using Alutard SQ Apis mellifera with alcohol
You should avoid alcohol on the day of injection, as it may increase the risk of suffering a severe allergic reaction (anaphylaxis).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Initiation of treatment with Alutard SQ Apis mellifera should not be started during pregnancy. If you become pregnant during maintenance treatment, you should discuss the risks of continuing maintenance treatment with your doctor.
It is not known whether Alutard SQ Apis mellifera is excreted in breast milk. You should consult your doctor before starting treatment if you are breastfeeding.
Driving and using machines
Alutard SQ Apis mellifera may affect your ability to drive or use machines, as you may feel dizzy after receiving the treatment.
Alutard SQ Apis mellifera contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use Alutard SQ Apis mellifera
Treatment with Alutard SQ Apis mellifera is administered by injection. The injections will normally be given in your arm, under the skin. Your doctor or nurse will always give you the injections.
You should remain in the clinic for at least 30 minutes after the injection to detect and treat any potential allergic reaction.
On the day of injection, you should avoid: intense physical exercise, hot baths, and alcohol.
The treatment is divided into two phases: initiation phase and maintenance phase.
Initiation phase:
Treatment will be started according to the schedule established by your doctor. In the initiation phase, it is usual for the injections to be given once a week. This phase usually lasts between 7 and 25 weeks.
The goal is to gradually increase the dose until the maximum dose that you can tolerate or the recommended maintenance dose is reached. If a reaction occurs at the injection site and lasts more than 6 hours after the injection, the doctor may adjust the dose based on the extent of your skin reaction. Your doctor may prescribe antihistamines before the injection.
Maintenance phase:
Once the maintenance dose is reached, the time between injections will be gradually increased, until they are given every 6-8 weeks for 3-5 years.
Treatment with more than one allergen at the same time:
If you are being treated with more than one allergen at the same time, the time between injections should be at least 30 minutes.
If you use more Alutard SQ Apis mellifera than you should
A doctor will administer the treatment with Alutard SQ Apis mellifera. In case of overdose, you will be monitored and treated by a doctor.
If you forget to use Alutard SQ Apis mellifera
Contact your doctor if you think you have missed a dose. If the time interval between 2 injections has been too long, the doctor will reduce the dose to avoid an allergic reaction.
If you stop using Alutard SQ Apis mellifera
To get the best result from your treatment, you will need to receive the injections for 3 to 5 years.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects can be an allergic reaction to the allergen being treated. Local reactions such as itching, redness, and swelling may occur at the injection site after each injection. Side effects usually occur within 30 minutes after the injection. However, late reactions can occur up to 24 hours after the injection.
Seek medical attention immediatelyif your asthma suddenly worsens or if you experience any of the following symptoms, which may be a sign of an anaphylactic reaction (the frequency cannot be estimated from the available data):
- Rapid swelling of the face or throat
- Difficulty swallowing
- Difficulty breathing
- Hives
- Flushing
- Worsening of existing asthma
- Nausea, abdominal pain, vomiting, and diarrhea
- Severe discomfort
Other possible side effects (the frequency cannot be estimated from the available data):
- Reactions at the injection site: swelling, nodules, pain, itching, redness, hair growth
- Headache
- Dizziness
- Itching sensation on the skin
- Swelling of the eyelids
- Inflammation or itching in the eyes
- Rapid heartbeat
- Sensation of rapid and strong or irregular heartbeat
- Low blood pressure
- Pallor
- Nasal congestion
- Feeling of oppression or irritation in the throat
- Wheezing
- Asthmatic symptoms, shortness of breath, or cough
- Rash
- Pain or swelling of the joints
- Feeling of heat
- Feeling of something stuck in the throat
- Swelling of the tissues (usually in the lower limbs)
- Discomfort in the chest
- Fatigue
- Feeling of discomfort
In case of any allergic reaction, you should contact your doctor immediately to receive the appropriate treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Alutard SQ Apis mellifera
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date is the last day of the month stated.
The vial should be used within 6 months after opening when used for a specific patient and stored in a refrigerator (between 2°C and 8°C).
Store in a refrigerator (between 2°C and 8°C). Do not freeze. Store in the original packaging to protect from light.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy. If you are unsure, ask your pharmacist how to dispose of the containers and any unused medicines. This will help protect the environment.
6. Contents of the pack and other information
Composition of Alutard SQ Apis mellifera
- The active substance is the allergen of bee venom (Apis mellifera).
- The other ingredients are aluminum hydroxide (hydrated), sodium chloride, sodium hydrogen carbonate, phenol, sodium hydroxide, human albumin solution, and water for injections.
Appearance of the product and pack contents
Alutard SQ Apis mellifera is an injectable suspension. The suspension is white to slightly brown or green in color.
The product is available in two presentations: a starter kit with four concentrations and a maintenance kit with a concentration of 100,000 SQ-U/ml. The numbers on the vials are of different colors to distinguish between the different concentrations. Not all pack sizes may be marketed.
Activity is expressed in SQ-U/ml units.
The activity in 1 ml of injectable suspension is:
Vial/ Color | Vial 1 Gray | Vial 2 Green | Vial 3 Orange | Vial 4 Red |
Concentration | 100 SQ-U | 1,000 SQ-U | 10,000 SQ-U | 100,000 SQ-U |
Aluminum content in the adjuvant | 0.00113 mg/ml | 0.0113 mg/ml | 0.113 mg/ml | 1.13 mg/ml |
Marketing authorization holder and manufacturer
Marketing authorization holder
ALK-Abelló A/S
Bøge Allé 6-8
2970 Hørsholm
Denmark
Manufacturer
ALK-Abelló S.A.
Miguel Fleta 19
28037 Madrid
Spain
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
ALK-Abelló S.A.
C/ Miguel Fleta, 19
28037 – Madrid
This medicine is authorized in the Member States of the European Economic Area and the United Kingdom (Northern Ireland) under the following names:
Belgium, Ireland, Luxembourg, United Kingdom (Northern Ireland) | ALUTARD SQ Bee |
Norway, Sweden | Alutard SQ Bigift |
Portugal, Spain | Alutard SQ Apis mellifera |
Austria | Alutard SQ Bienengift |
France | ALUTARD Venom d'abeille APIS MELLIFERA |
Hungary | Alutard SQ Méh |
Italy | Alutard Apis mellifera |
Romania | Alutard SQ venin de albina |
Slovenia | Cebelji strup Alutard SQ |
Date of last revision of this leaflet:07/2025
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
This information is intended only for healthcare professionals:
Treatment with Alutard SQ Apis mellifera should be performed under the supervision of a doctor with experience in specific immunotherapy. After each injection, the patient should remain under observation for at least 30 minutes.
During storage, a precipitate and a clear liquid may be observed. This is normal in a suspension and does not constitute a sign of deterioration of the product quality. The precipitate may be white to slightly brown or green in color. The vials should be inverted slowly up and down 10-20 times to form a homogeneous suspension before use. Visually inspect the suspension for fine particles before administration. Discard the product if there are visible particles in it.
Alutard SQ Apis mellifera is administered subcutaneously. The injection is given either laterally in the distal part of the upper arm or dorsally in the proximal part of the forearm.
Avoid intravascular injection by carefully aspirating before injection. Aspiration should be repeated every 0.2 ml during injection, and the injection should be given slowly. During the use of Alutard SQ Apis mellifera, an emergency kit should be available to treat anaphylaxis.
Due to the lack of compatibility studies, this medicine should not be mixed with others.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALUTARD SQ APIS MELLIFERA Starter Pack (100 SQ-U/mL, 1,000 SQ-U/mL, 10,000 SQ-U/mL and 100,000 SQ-U/mL) Injectable SuspensionDosage form: INJECTABLE, 100,000 IU/mlActive substance: insectsManufacturer: Alk-Abello A/SPrescription requiredDosage form: INJECTABLE, 100,000 IU/mlActive substance: insectsManufacturer: Alk-Abello A/SPrescription requiredDosage form: INJECTABLE, 100 IU/ml + 1,000 IU/ml + 10,000 IU/ml + 100,000 IU/mlActive substance: insectsManufacturer: Alk-Abello A/SPrescription required
Online doctors for ALUTARD SQ APIS MELLIFERA Starter Pack (100 SQ-U/mL, 1,000 SQ-U/mL, 10,000 SQ-U/mL and 100,000 SQ-U/mL) Injectable Suspension
Discuss questions about ALUTARD SQ APIS MELLIFERA Starter Pack (100 SQ-U/mL, 1,000 SQ-U/mL, 10,000 SQ-U/mL and 100,000 SQ-U/mL) Injectable Suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















