AGAMREE 40 mg/mL ORAL SUSPENSION

How to use AGAMREE 40 mg/mL ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
AGAMREE 40 mg/ml Oral Suspension
vamorolone
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is AGAMREE and what is it used for
- What you need to know before you take AGAMREE
- How to take AGAMREE
- Possible side effects
- Storing AGAMREE
- Contents of the pack and other information
1. What is AGAMREE and what is it used for
AGAMREE is a steroid anti-inflammatory medicine that contains the active substance vamorolone.
AGAMREE is used to treat patients from 4 years of age with Duchenne muscular dystrophy (DMD). DMD is a genetic disease caused by defects in the dystrophin gene, which normally produces a protein that keeps muscles healthy and strong. In patients with DMD, this protein is not produced and the body is unable to develop new muscle cells or replace damaged muscle. This causes the muscles in the body to weaken over time.
AGAMREE is used to stabilize or improve muscle strength in patients with DMD.
2. What you need to know before you take AGAMREE
Do not take AGAMREE
- if you are allergic to vamorolone or any of the other ingredients of this medicine (listed in section 6);
- if you have severe liver problems;
- if you are scheduled to receive or have received any live or attenuated vaccinations (against measles, mumps, rubella, or chickenpox) in the last 6 weeks. Consult your doctor if you are already taking AGAMREE and are planning to get vaccinated.
Warnings and precautions
Talk to your doctor before you start taking AGAMREE
Endocrine disorders: adrenal insufficiency
AGAMREE reduces the amount of a hormone called cortisol that your body can produce. This is called adrenal insufficiency.
- do not reduce the dose or stop taking AGAMREE without talking to your doctor; if you reduce or stop taking AGAMREE abruptly for a few days, you may develop symptoms of acute adrenal insufficiency, such as excessive tiredness, dizziness, or confusion, which can be life-threatening; your doctor may need to monitor your treatment more closely if you change the dose.
- if you experience unusual stress (such as acute infection, traumatic injuries, or major surgery), you may need to take another steroid medicine to prevent acute adrenal insufficiency. Talk to your doctor about what to do in case of unusual stress before starting treatment with AGAMREE.
- if you are taking another corticosteroid, such as prednisone, you may be able to switch to AGAMREE from one day to the next, but your doctor will tell you what dose of AGAMREE to take.
- if you have a type of tumor in the adrenal glands called pheochromocytoma, your doctor may need to monitor your treatment more closely
IMPORTANT: The AGAMREE package includes a patient alert card that contains important safety information about adrenal crises. Always carry this card with you.
Weight gain
- AGAMREE may increase your appetite and, therefore, your weight, mainly during the first few months of treatment; your doctor or nurse will give you dietary advice before and during treatment.
Patients with altered thyroid function
- if you have hypothyroidism (underactive thyroid) or hyperthyroidism (overactive thyroid), your doctor may need to monitor your treatment more closely or change your dose.
Ophthalmic effects
- if you or a family member has glaucoma (increased eye pressure), your doctor may need to monitor your treatment more closely.
Increased risk of infections
AGAMREE may reduce your natural resistance to infections.
- if you have a reduced immune response (due to an immunodeficiency syndrome, disease, or other medicines that suppress the immune system), your doctor may need to monitor your treatment more closely;
- if you experience an infection while taking AGAMREE, your doctor may need to monitor you more closely and may need to give you additional steroid treatment.
Diabetes mellitus
- Over time, the use of AGAMREE may increase the likelihood of developing diabetes mellitus (a sugar-related disease); your doctor may check your sugar levels regularly.
Vaccination
- if you are scheduled to receive a vaccination with live or attenuated vaccines, you should be vaccinated at least 6 weeks before starting treatment with AGAMREE.
- if you have never had chickenpox or have not been vaccinated against chickenpox, you may want to discuss vaccination with your doctor before starting treatment with AGAMREE.
Thromboembolic episodes
- if you have had thromboembolic episodes (a blood clot inside the body) or a disease that increases the risk of blood clotting, your doctor may need to monitor your treatment more closely.
Liver failure
- if you have liver disease, your doctor may need to change the dose.
Children
Do not give AGAMREE to children under 4 years of age, as this medicine has not been tested in this group of patients.
Other medicines and AGAMREE
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you are taking any of the following medicines:
- Medicines used to treat seizures and neuropathic pain, such as carbamazepine or phenytoin, as they may affect the effect of the medicine.
- Medicines used to treat fungal infections (including candidiasis and aspergillosis) known as triazoles, such as itraconazole and voriconazole, as they may affect the effect of the medicine.
- Antibiotics known as macrolides (such as clarithromycin) or "cetolides" (such as telithromycin), as they may affect the effect of the medicine.
- Antibiotics known as rifamycins, such as rifampicin, as they may affect the effect of the medicine.
- Spironolactone or eplerenone, known as potassium-sparing diuretics (treatments that increase urine production), which may be used to lower blood pressure and protect cardiovascular function; your doctor may need to monitor your potassium levels and change the dose of these medicines.
- St. John's Wort (Hypericum perforatum), a herbal medicine used to treat depression and emotional disorders, as it may affect the effect of the medicine.
If you need to receive a vaccine, consult your doctor first (see section 2: "Do not take AGAMREE"). If you are going to be vaccinated with certain types of vaccines (live or attenuated vaccines), you should be vaccinated at least 6 weeks before starting treatment with AGAMREE, as these vaccines may trigger the infection they are supposed to prevent.
AGAMREE with food and drinks
Avoid grapefruit and grapefruit juice during treatment with AGAMREE, as they may affect the effect of the medicine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
If you are pregnant, you should not take AGAMREE unless your doctor tells you to.
If you are a woman who could become pregnant, you should use effective birth control methods during treatment with AGAMREE.
Animal studies have shown that long-term treatment with AGAMREE may affect male and female fertility.
Driving and using machines
Consult your doctor if the disease you are suffering from allows you to drive vehicles, including bicycles, and use machines safely. AGAMREE is not expected to affect your ability to drive, ride a bicycle, or use machines.
AGAMREE contains sodium benzoate and sodium
AGAMREE contains 1 mg of sodium benzoate (E211) per ml.
AGAMREE contains less than 23 mg of sodium per 7.5 ml; this is, essentially, "sodium-free".
3. How to take AGAMREE
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist.
The recommended dose of AGAMREE depends on your body weight and age.
If the patient is 4 years or older and weighs less than 40 kg, the dose is usually 6 mg per kg of body weight, given once a day.
If the patient is 4 years or older and weighs 40 kg or more, the dose is usually 240 mg, taken once a day.
If you experience certain side effects while taking AGAMREE (see section 4), your doctor may reduce the dose or interrupt treatment temporarily or permanently. Your doctor may reduce the dose if you have liver disease.
This medicine is taken by mouth. AGAMREE can be taken with or without food (see section 2 "AGAMREE with food and drinks").
To extract the medicine, use one of the oral syringes included in the package. Use only these oral syringes to measure the dose. Your doctor will tell you how much to extract with the syringe for your daily dose.
Caregivers should assist with the administration of AGAMREE, especially when it comes to using oral syringes to measure and administer the prescribed dose.
Shake the bottle well before extracting the medicine with the syringe. Fill the oral syringe with the prescribed dose and then slowly pour the contents of the syringe into the mouth. Read the instructions below for more information on how to measure and take the dose correctly. Consult your doctor or pharmacist if you are not sure how to use the oral syringe.
After taking the prescribed dose, disassemble the syringe, wash the syringe and plunger under cold running water, and dry it in the air. Keep the syringe clean in the package until the next use. The oral syringe should only be used for a maximum of 45 days. After this time, discard it and use the second syringe included in the package. If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
HOW TO PREPARE THE DOSE OF AGAMREE ORAL SUSPENSION
Before taking/administering AGAMREE | ||
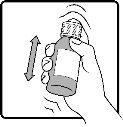
Step 1 | Make sure the child-resistant cap is properly protected and shake the bottle well. |
|
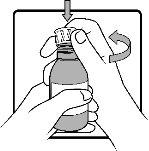
Step 2 | Remove the child-resistant cap by pressing it firmly downwards and turning it counterclockwise. |
|
Step 3 | Firmly insert the adapter into the bottle. This should be done the first time you open the bottle. The adapter will then remain in the bottle. If the adapter falls out, wash it with cold running water and dry it in the air for at least 2 hours. |
|
Preparing a dose of AGAMREE | ||
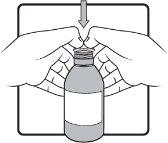
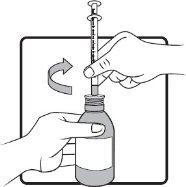
Step 4 | Keep the bottle in a vertical position. Before inserting the tip of the oral syringe into the adapter, press the plunger down until it stops at the tip of the syringe. Insert the tip firmly into the adapter opening. |
|
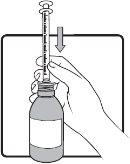
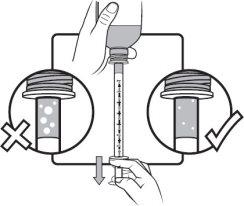
Step 5 | Keep the syringe in place and carefully turn the bottle upside down. Slowly pull the plunger until the desired amount of medicine is drawn into the syringe. If there are large air bubbles in the syringe (as shown in the figure on the left) or if you have drawn an incorrect dose of AGAMREE, insert the tip of the syringe firmly into the adapter while the bottle is in a vertical position. Push the plunger down to return AGAMREE to the bottle and repeat steps 4 to 6. |
|
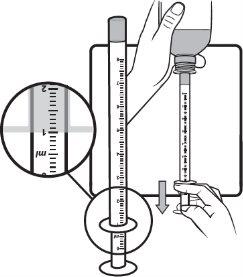
Step 6 | Check the dose in milliliters (ml) as prescribed by your doctor. Find the graduation to read the dose in milliliters (ml) on the plunger, as shown in the image on the right. On the scale represented, each line corresponds to 0.1 ml. In the example, a dose of 1 ml is shown. Do not take more than the prescribed daily dose. |
|
Step 7 | Turn the bottle to its right side and carefully remove the syringe from the bottle. Do not hold the syringe by the plunger, as it may come off. |
|
Administration of AGAMREE | ||
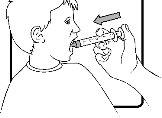
Step 8 | Do not mix the medicine with any liquid before administration. The patient should be in an upright position when taking the medicine. Empty the syringe directly into the mouth. Gently press the plunger to empty the syringe. Do not push it forcefully. To avoid the risk of choking, make sure the medicine does not go directly to the back of the mouth or throat. |
|
After administering AGAMREE | ||
Step 9 | Close the bottle with the child-resistant cap after each use. | |
Step 10 | Disassemble the oral syringe, rinse it with cold running water, and dry it in the air before its next use. Each oral syringe supplied with AGAMREE can be used for a maximum of 45 days. |
If you take more AGAMREE than you should
If you take too much AGAMREE, contact your doctor or a hospital for advice. Show the AGAMREE package and this leaflet. Medical treatment may be necessary.
If you forget to take AGAMREE
Do not take more AGAMREE and do not repeat the dose.
Take the next dose as you would normally.
Talk to your healthcare professional if you are concerned.
If you stop taking AGAMREE
Take AGAMREE for as long as your doctor tells you. Consult your doctor before stopping treatment with AGAMREE, as it is necessary to gradually reduce the dose to avoid adverse reactions.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Treatment with AGAMREE causes adrenal insufficiency. Consult your doctor before starting to take AGAMREE (see section 2 for more information).
The following adverse effects have been reported with AGAMREE with a category of very frequent (may affect more than 1 in 10 people):
- Swollen and more rounded face appearance (Cushing's syndrome)
- Weight gain (weight increase)
- Increased appetite
- Irritability
- Vomiting
The following adverse effects have been reported with a frequency category (may affect up to 1 in 10 people):
- Abdominal pain (abdominal pain)
- Pain in the upper abdomen (upper abdominal pain)
- Diarrhea
- Headache
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of AGAMREE
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and on the label of the vial after "EXP". The expiration date is the last day of the indicated month.
This medicine does not require any special storage temperature.
After opening AGAMREE for the first time, store the vial in a vertical position in the refrigerator (between 2 °C and 8 °C). The medicine can be stored in the refrigerator for a maximum of 3 months.
Discard the unused medicine within 3 months of the first opening of the vial.
Medicines should not be thrown away through drains or into the trash. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This way, you will help protect the environment.
6. Package Contents and Additional Information
What AGAMREE contains
The active ingredient is vamorolone. Each ml of suspension contains 40 mg of vamorolone.
The other components are: citric acid (monohydrate) (E 330), disodium phosphate (E 339), glycerol (E 422), orange flavor, purified water, sodium benzoate (E 211) (see section 2, "AGAMREE contains sodium benzoate"), sucralose (E 955), xanthan gum (E 415), and hydrochloric acid (for pH adjustment). See section 2 "AGAMREE contains sodium benzoate and sodium". Note: There are no image placeholders in the provided HTML code, so there is nothing to translate in that regard. The translation provided is complete and accurate, following the provided guidelines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AGAMREE 40 mg/mL ORAL SUSPENSIONDosage form: INJECTABLE, 373 mgActive substance: hydrocortisoneManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 75 mgActive substance: hydrocortisoneManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 0.5 mgActive substance: hydrocortisoneManufacturer: Diurnal Europe B.V.Prescription required
Online doctors for AGAMREE 40 mg/mL ORAL SUSPENSION
Discuss questions about AGAMREE 40 mg/mL ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions