
ACTUATOS 35 mg ORAL SOLUTION IN SACHETS

How to use ACTUATOS 35 mg ORAL SOLUTION IN SACHETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Actúatos 35 mgsyrup in sachets
dry extract ofHedera helix L.(ivy)
Read the entire leaflet carefully before starting to take this medication because it contains important information for you.
Follow the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist exactly.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 7 days.
Contents of the leaflet
- What is actúatos and what is it used for
2.
- How to take actúatos
4.
- Storage of actúatos
6.
1. What is actúatos and what is it used for
Actúatos 35 mg syrup in sachets is a plant-based medication used as an expectorant for productive cough in adults, adolescents, and children from 6 years old.
2. What you need to know before taking actúatos
Do not take actúatos:
- If you are allergic to ivy (Hedera helix L.), to plants of the Araliaceae family, or to any of the other components of this medication (listed in section 6).
- Do not administer to children under 2 years old, as there is a risk that expectorants may worsen respiratory symptoms.
Warnings and precautions:
Consult your doctor or pharmacist before starting to take this medication.
You should consult with your doctor or pharmacist in cases of dyspnea (difficulty breathing), fever, or purulent sputum.
Cautions are recommended in patients with gastritis or gastric ulcers.
In case of worsening symptoms or if no improvement occurs after 7 days of starting treatment, it should be discontinued and a doctor should be consulted.
Children
This medication should not be administered to children from 2 to 5 years old, as it cannot be dosed adequately; there are other formulations more suitable for this age group.
Taking actúatos with other medications:
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
Pregnancy, breastfeeding, and fertility:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy and breastfeeding:
The safety of this medication during pregnancy and breastfeeding has not been established; therefore, its use is not recommended in these circumstances.
Fertility:
No data are available on fertility.
Consult your doctor or pharmacist before using any medication.
Driving and using machines
No studies have been conducted on the effects on the ability to drive and use machines.
Actúatoscontains sorbitol.
This medication contains 1.925 mg of sorbitol in each 5 ml sachet (385 mg per ml).
Sorbitol is a source of fructose. If your doctor has told you that you (or your child) have an intolerance to certain sugars or have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disease in which the patient cannot break down fructose, consult your doctor before taking this medication.
Sorbitol may cause gastrointestinal upset and a mild laxative effect.
3. How to take actúatos
Follow the administration instructions of this medication contained in this leaflet or as indicated by your doctor or pharmacist exactly. If in doubt, ask your doctor or pharmacist.
The recommended dose is:
- Adolescents and adults: 1 sachet of 5 ml of syrup, 2 or 3 times a day (equivalent to 70-105 mg daily of dry extract of ivy leaf).
- Children between 6 and 11 years old:1 sachet of 5 ml of syrup, 2 times a day (equivalent to 70 mg daily of dry extract of ivy leaf).
This medication should not be administered to children from 2 to 5 years old, as it cannot be dosed adequately; there are other formulations more suitable for this age group.
If you think the action of this medication is too strong or too weak, tell your doctor or pharmacist.
This medication is taken orally.
For more details on using the sachets, follow the diagrams below:
Press the sachet gently before using it, as shown.
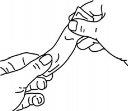
Hold the sachet firmly and tear it along the indicated lines.
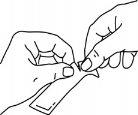
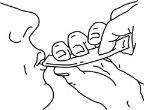
Swallow the medication, squeezing the sachet until it is empty.
You should consult a doctor if it worsens or does not improve after a week of treatment.
If you take more actúatosthan you should
Overdose may cause nausea, vomiting, diarrhea, and agitation.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, indicating the medication and the amount ingested.
If you forget to takeactúatos:
Do not take a double dose to make up for the forgotten dose.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone may experience them.
Unknown frequency: gastrointestinal-type reactions such as nausea, vomiting, and diarrhea, and allergic reactions such as hives, skin rashes, difficulty breathing, and anaphylactic reaction have been reported.
If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of actúatos
Keep this medication out of the sight and reach of children.
No special storage conditions are required.
Do not use this medication after the expiration date shown on the packaging, after "EXP". The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of actúatos 35 mg syrup in sachets:
- The active ingredient is: dry extract of Hedera helixleaf.
5 ml of actúatos 35 mg syrup in sachets contains 35 mg of dry extract of Hedera helix L. leaf (4-8:1), extraction solvent: ethanol 30% m/m.
- The other components are: purified water, potassium sorbate, citric acid, non-crystallizable liquid sorbitol (E-420), xanthan gum, and cherry flavor. See section 2 "Actúatos contains sorbitol"
Appearance of the product and package contents:
Actúatos 35 mg syrup in sachets is presented in sachets containing 5 ml of syrup.
Monodose sachets composed of a complex aluminum layer (PET/Aluminum/PE/PET/PE). Packages with 20 monodose sachets.
Marketing authorization holder and manufacturer:
Marketing authorization holder
Adventia Pharma, S.L.
Calle Viera y Clavijo, 30, 2º
35002 Las Palmas de Gran Canaria (Spain)
Manufacturer:
Kern Pharma S.L.
Venus, 72 - Pol. Ind. Colón II
08228 Terrassa - Barcelona
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Actúa Pharma, S.L.
Calle Viera y Clavijo, 30, 2º
35002 Las Palmas de Gran Canaria (Spain)
Date of the last revision of this leaflet: June 2025
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACTUATOS 35 mg ORAL SOLUTION IN SACHETSDosage form: ORAL SOLUTION/SUSPENSION, 7 mg/mlActive substance: Hederae helicis foliumManufacturer: Engelhard Arzneimittel Gmbh & Co. KgPrescription not requiredDosage form: ORAL SOLUTION/SUSPENSION, 7 mg/mlActive substance: Hederae helicis foliumManufacturer: Adventia Pharma S.L.Prescription not requiredDosage form: ORAL SOLUTION/SUSPENSION, 8.25 mg/mlActive substance: Hederae helicis foliumManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for ACTUATOS 35 mg ORAL SOLUTION IN SACHETS
Discuss questions about ACTUATOS 35 mg ORAL SOLUTION IN SACHETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







