
ACALKA 1080 mg PROLONGED-RELEASE TABLETS

How to use ACALKA 1080 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Acalka 1080 mg
prolonged-release tablets
Potassium citrate
Contents of the leaflet
- What is Acalka and what is it used for
- What you need to know before taking Acalka
- How to take Acalka
- Possible side effects
5 Conservation of Acalka
- Package contents and additional information
1. What is Acalka and what is it used for
Acalka belongs to the group of medicines called urinary concretion solvents that serve to dissolve the stones (kidney stones) of the urinary system.
Acalka reduces the formation of calcium oxalate crystals and the precipitation of uric acid. It increases the pH of the urine, attempting to restore its normal levels without causing any alteration in the body.
It is indicated in the prevention and treatment of Lithiasis in the kidney (formation of stones or kidney stones) due to calcium oxalate and/or calcium phosphate, uric acid alone or accompanied by calcic lithiasis and hypocitraturia (decrease in citrates in the urine).
2. What you need to know before taking Acalka
Do not take Acalka
- If you are allergic (hypersensitive) to potassium citrate or any of the other components of this medicine (included in section 6).
- If you have renal insufficiency (kidney disease) since the use of Acalka could produce hyperkalemia (increase in potassium levels in the blood).
hyperkalemia (increase in potassium levels in the blood).
- If you have a permanent alkaline urinary tract infection.
- If you have an obstruction in the urinary system (partial or total blockage of the urinary system that produces an interruption of the passage of urine through it).
produces an interruption of the passage of urine through it).
- If you have hyperkalemia (increase in potassium levels in the blood).
- If you have adrenal insufficiency (adrenal gland disease that leads to a hormonal deficiency).
a hormonal deficiency).
- If you have respiratory alkalosis (decrease in carbon dioxide levels in the blood) or metabolic alkalosis (increase in bicarbonate levels in the blood).
- If you have an active peptic ulcer (stomach or duodenal ulcer).
- If you have an intestinal obstruction (partial or total blockage of the intestine that produces an interruption of the passage of intestinal contents through it).
through it).
- If you have a slow gastric emptying (emptying of the stomach).
- If you are taking anticholinergic medications.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Acalka.
- If you have liver failure (liver disease) or a decrease in potassium excretion (elimination by the body) since the use of Acalka could produce hyperkalemia (increase in potassium levels in the blood).
hyperkalemia (increase in potassium levels in the blood).
- Potassium citrate is included in wax matrix tablets that allow the slow release of potassium citrate. It is likely that once the wax matrix is empty, it will be eliminated intact through the stool and still be visible.
still be visible.
Using Acalka with other medicines
Tell your doctor or pharmacist that you are using, have recently used, or may need to use any other medicine.
Acalka should not be taken with potassium-sparing diuretic medications (medicines used to increase urine elimination without increasing potassium elimination) such as Triamterene, Spironolactone, or Amiloride.
Using Acalka with food and beverages
While taking Acalka, it is recommended to follow a salt-free diet and significantly increase daily fluid intake.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
If you are pregnant, take Acalka only if your doctor has indicated it.
Do not take Acalka if you are breastfeeding unless your doctor has indicated it.
Driving and using machines
The influence on the ability to drive or use machines is nil.
3. How to take Acalka
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Mild or moderate hypocitraturia (decrease in citrates in the urine):
The usual dose of Acalka is 1 tablet (10 mEq of potassium citrate) 3 times a day, 30 minutes after the 3 main meals.
Severe hypocitraturia (decrease in citrates in the urine):
The usual dose of Acalka is 2 tablets (20 mEq of potassium citrate) 3 times a day, 30 minutes after the 3 main meals.
Do not exceed, even in very severe cases of hypocitraturia, 10 tablets.
Acalka tablets are for oral administration.
Swallow the tablets whole with a sufficient amount of liquid, without chewing, breaking, or dissolving in a liquid, 30 minutes after the 3 main meals to prevent the appearance of mild gastrointestinal disorders (stomach and intestine).
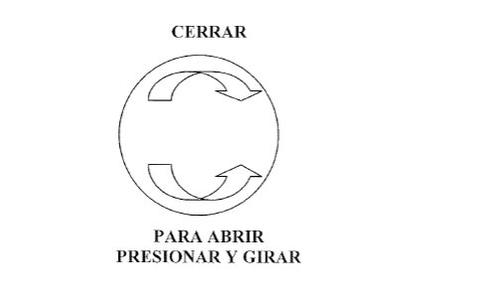
Open the bottle by following the instructions indicated below:
If you take more Acalka than you should
If you have taken more Acalka than you should, consult your doctor or pharmacist immediately. In case of overdose or accidental ingestion, also consult the Toxicology Information Service, phone (91) 562.04.20.
In case of hyperkalemia (increase in potassium levels in the blood), the treatment consists of administering an intravenous solution of 10% dextrose with 10-12 U of insulin/1000 ml. If acidosis is also present, the treatment consists of administering intravenous sodium bicarbonate and hemodialysis or peritoneal dialysis.
If you forget to take Acalka
Do not take a double dose to make up for forgotten doses.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Acalka can have side effects, although not everyone gets them.
Acalka can produce mild gastrointestinal disorders (stomach and intestine) that decrease if taken with food.
If you experience side effects, consult your doctor or pharmacist, even if they are side effects that do not appear in this leaflet.
5. Conservation of Acalka
Keep this medicine out of sight and reach of children.
Keep the bottle perfectly closed to protect it from light and moisture.
Do not use this medicine after the expiration date that appears on the packaging and label after CAD. The expiration date is the last day of the month indicated.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Acalka
- The active ingredient is potassium citrate. Each tablet contains 1080 mg of potassium citrate (10 milliequivalents) which corresponds to 390 mg of potassium.
- The other components (excipients) are: Carnauba wax (E-903) and Magnesium stearate.
Appearance of the product and package contents
Acalka is a prolonged-release tablet. It is presented in packages of 100 tablets.
Marketing authorization holder and manufacturer
Holder:
Ferrer Internacional, S.A.
Gran Vía Carlos III, 94
08028-Barcelona
Manufacturer:
Ferrer Internacional, S.A.
Joan Buscallà, 1-9
08173 Sant Cugat del Vallès (Barcelona)
Date of the last revision of this leaflet:July 2012
“Detailed and updated information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/”
- Country of registration
- Average pharmacy price26.35 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACALKA 1080 mg PROLONGED-RELEASE TABLETSDosage form: TABLET, 25 mgActive substance: sildenafilManufacturer: Adamed Pharma S.A.Prescription requiredDosage form: TABLET, 10 mgActive substance: tadalafilManufacturer: Gp Pharm S.A.Prescription requiredDosage form: TABLET, 20 mgActive substance: tadalafilManufacturer: Gp Pharm S.A.Prescription required
Online doctors for ACALKA 1080 mg PROLONGED-RELEASE TABLETS
Discuss questions about ACALKA 1080 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







