
ABFENTIQ 600 micrograms LOZENGE tablets

How to use ABFENTIQ 600 micrograms LOZENGE tablets
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Abfentiq 200 micrograms buccal tablets EFG
Abfentiq 400 micrograms buccal tablets EFG
Abfentiq 600 micrograms buccal tablets EFG
Abfentiq 800 micrograms buccal tablets EFG
Abfentiq 1200 micrograms buccal tablets EFG
Abfentiq 1600 micrograms buccal tablets EFG
Fentanyl
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Abfentiq and what is it used for
- What you need to know before you use Abfentiq
- How to use Abfentiq
- Possible side effects
5 Storage of Abfentiq
- Contents of the pack and further information.
1. What is Abfentiq and what is it used for
Abfentiq contains the active substance fentanyl, a potent analgesic belonging to the group of opioids. Abfentiq is presented as buccal tablets with an integrated applicator.
- Abfentiq is indicated for the treatment of breakthrough pain in adult patients with cancer, who are already taking other opioid medicines for their persistent (chronic) cancer pain. Breakthrough pain is a sudden increase in pain that occurs in addition to the persistent pain, and that is not relieved by the opioid medicines used to treat the persistent pain.
- Do not use Abfentiq if you have not been taking opioid medicines every day for at least one week to control your persistent pain, as the use of Abfentiq may increase the risk of your breathing becoming slower and/or shallower, and even stopping.
- Do not use Abfentiq to treat pain caused by wounds, surgery, headache, or migraines.
2. What you need to know before you use Abfentiq
Do not use Abfentiq
- if you are not already using a prescribed opioid medicine every day, at the same time, for at least one week, to control your persistent pain. If you have not been using these medicines, do not use Abfentiq as its use may increase the risk of your breathing becoming slower and/or shallower, and even stopping
- if you are allergic (hypersensitive) to fentanyl or any of the other ingredients of Abfentiq (listed in Section 6).
- if you are currently taking monoamine oxidase inhibitors (MAOIs) for severe depression (or have taken them in the last 2 weeks).
- if you have severe respiratory problems or severe obstructive pulmonary disease.
- if you have short-term pain that is not breakthrough pain
Do not use Abfentiq if you are in any of the above situations. If you are not sure, consult your doctor or pharmacist before using Abfentiq.
Warnings and precautions:
During treatment with Abfentiq, continue to use the opioid pain medicine you are taking for your persistent (chronic) cancer pain.
If you have any of the following conditions, consult your doctor or pharmacist before using Abfentiq:
- if the other opioid medicine you are taking for your persistent pain is not yet stable.
- if you have any disease that affects your breathing (such as asthma, wheezing, or shortness of breath).
- if you have had a head injury or have lost consciousness.
- if you have heart problems, especially a low heart rate.
- if you have liver or kidney problems, as these affect how the body eliminates the medicine.
- if you have low blood pressure due to low fluid volume in the circulatory system.
- if you are diabetic.
- if you are over 65 years old, as the dose may need to be reduced. Any increase in dose should be carefully supervised by your doctor.
- if you are taking antidepressants or antipsychotics; see the section “Using other medicines”.
- if you are taking pain medicines for nerve pain (gabapentin and pregabalin).
It is possible that your doctor will need to monitor you more closely:
- if you or a family member have ever had problems with alcohol, prescription medicine, or drug abuse (“addiction”).
- if you are a smoker.
- if you have ever had problems related to your mood (depression, anxiety, or personality disorder) or have been treated by a psychiatrist for other mental illnesses.
Talk to your doctor if, DURING the use of Abfentiq:
- you feel pain or increased sensitivity to pain (hyperalgesia) that does not respond to a higher dose of the medicine as prescribed by your doctor.
- you experience a combination of the following symptoms: nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure. Together, these symptoms can be a sign of a potentially life-threatening condition called adrenal insufficiency, in which the adrenal glands do not produce enough hormones.
- you have ever had adrenal insufficiency or a lack of sex hormones (androgen deficiency) with the use of opioids.
- if you experience respiratory disorders related to sleep: Abfentiq may cause sleep-related respiratory disorders such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen level in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty staying asleep, or excessive daytime sleepiness. If you or someone else observes these symptoms, contact your doctor. Your doctor may consider reducing the dose.
- Repeated use of Abfentiq can lead to dependence and abuse, which can cause a potentially life-threatening overdose. If you are concerned about the possibility of becoming dependent on Abfentiq, it is essential that you consult your doctor.
Children and adolescents
Abfentiq is not recommended for children under 16 years of age.
Use in athletes
This medicine contains fentanyl, which can produce a positive result in doping tests.
Using Abfentiq with other medicines
Tell your doctor or pharmacist if you are using or have recently used any of the following medicines:
- Other fentanyl-based treatments that your doctor has previously prescribed for breakthrough pain. If you still have these fentanyl products at home, contact your pharmacist, who will tell you how to dispose of them.
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those bought without a prescription and herbal remedies. In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- Any medicine that can make you sleepy, such as sleeping pills, medicines for anxiety, or certain medicines for allergies (antihistamines) or tranquilizers.
- Certain muscle relaxants (such as baclofen or diazepam).
- Any medicine that can affect how the body eliminates Abfentiq, such as ritonavir or other medicines used to control HIV infection (AIDS) or other medicines called “CYP3A4 inhibitors” like ketoconazole, itraconazole, or fluconazole (used for fungal infections) and troleandomycin, clarithromycin, or erythromycin (medicines for bacterial infections) and “CYP3A4 inducers” like rifampicin or rifabutin (medicines for bacterial infections), carbamazepine, phenobarbital, or phenytoin (medicines used to treat seizures/attacks)
- Any medicine that can reduce or reverse the effect of Abfentiq (such as naloxone, pentazocine, buprenorphine). They can cause withdrawal syndrome.
- If you need to undergo surgery that requires general anesthesia.
- The risk of side effects increases if you are taking medicines such as certain antidepressants or antipsychotics. Abfentiq can interact with these medicines, and you may experience changes in mental status (e.g., agitation, hallucinations, coma) and other effects such as body temperature above 38°C, increased heart rate, unstable blood pressure, and exaggerated reflexes, muscle stiffness, lack of coordination, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Your doctor will tell you if Abfentiq is suitable for you.
Using Abfentiq with sedatives:
The concomitant use of Abfentiq and sedative medicines, such as benzodiazepines or related medicines, increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can be life-threatening. Therefore, concomitant use should only be considered when there are no other possible treatment alternatives.
However, if your doctor has prescribed Abfentiq and sedatives at the same time, the dose and duration of treatment should be limited by your doctor.
Tell your doctor about all sedatives you are taking and strictly follow the dose recommended by your doctor. It may be helpful to inform your family or friends about the signs and symptoms mentioned above. Talk to your doctor if you experience any of these symptoms.
Using Abfentiq with food, drinks, and alcohol
- Abfentiq can be used before or after meals. However, do not use it during a meal.
- You can drink a little water before using Abfentiq to moisten your mouth. However, do not drink or eat anything while using Abfentiq.
- Do not drink grapefruit juice while using Abfentiq, as it may affect how the body eliminates Abfentiq.
- Do not drink alcoholic beverages while being treated with Abfentiq, as it may increase the risk of serious side effects.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Abfentiq during childbirth, as it may cause breathing difficulties in the newborn. There is also a risk that the newborn may experience withdrawal syndrome if Abfentiq has been used for a long time during pregnancy.
Fentanyl can pass into breast milk and cause side effects in the baby. Do not use Abfentiq if you are breastfeeding. You should not start breastfeeding until at least 48 hours after the last dose of Abfentiq.
Consult your doctor or pharmacist before using any medicine if you are pregnant or breastfeeding.
Driving and using machines
This medicine can affect your ability to drive or operate certain tools or machinery. Consult your doctor about the safety for you to drive or operate certain tools or machinery in the hours following the use of Abfentiq.
Do not drive or operate certain tools or machinery if: you feel drowsy or dizzy; you have blurred or double vision; you have difficulty concentrating. It is essential that you know how Abfentiq affects you before driving or operating certain tools or machinery.
Abfentiq contains glucose and sodium.
- This medicine contains glucose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine. It may cause cavities.
This medicine contains less than 23 mg of sodium (1mmol) per buccal tablet; this is, essentially “sodium-free”.
3. How to use Abfentiq
Follow exactly the administration instructions of this medication indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
When you start using Abfentiq for the first time, your doctor will work with you to find the Abfentiq dose that relieves your breakthrough pain. It is very important that you use Abfentiq exactly as your doctor has indicated.
- Do not change the doses of Abfentiq or other analgesics on your own. Any change in dosage must be prescribed and monitored by your doctor.
- If you have doubts about the correct dose or if you have questions about the use of Abfentiq, consult your doctor.
How the medication penetrates the body
When you put Abfentiq in your mouth:
- The lozenge dissolves, and the drug is released. This process takes place in about 15 minutes.
- The drug is absorbed through the oral mucosa into the bloodstream.
The fact of using the medication in this way allows it to be absorbed quickly, which means rapid relief of breakthrough pain.
Determination of the correct dose
You should start to feel relief quickly while using Abfentiq. However, until you and your doctor determine the dose that effectively controls the breakthrough pain, you may not feel sufficient pain relief 30 minutes after starting to use a unit of Abfentiq (15 minutes after you finish using the Abfentiq lozenge). If this happens, your doctor may allow you to use a second Abfentiq lozenge of the same dose to treat the same episode of breakthrough pain.
Do not use a second unit unless your doctor indicates it.
Never use more than two units to treat a single episode of breakthrough pain.
During the determination of the correct dose, you may need to have units of Abfentiq of different concentrations at home. However, keep at home only the concentrations of Abfentiq that you need. This helps prevent possible confusion and overdose. Consult with the pharmacist how to dispose of the units of Abfentiq that you do not need.
How many units should be used
Once you have determined the correct dose with your doctor, use 1 unit for an episode of breakthrough pain. Consult with your doctor if your correct dose of Abfentiq does not relieve the breakthrough pain over several consecutive episodes of breakthrough pain. Your doctor will decide if it is necessary to modify the dose.
You must inform your doctor immediately if you use Abfentiq more than four times a day. In that case, it may be convenient to change the medication for persistent pain (present all the time). Once this is done, when the persistent pain is controlled, the doctor may need to change the dose of Abfentiq again. To get better results, inform your doctor about the pain you are experiencing and how Abfentiq is working. This way, the dose can be changed if necessary.
Use of the medication
Opening the packaging
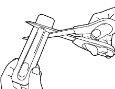
- Each unit of Abfentiq is sealed in its own blister packaging.
Do not open the packaging ahead of time.
- Hold the blister packaging with the printed side opposite you.
- Hold the short end of the blister packaging.
- Place the scissors near the end of the Abfentiq unit and cut completely the long end (see the illustration).
- Separate the printed back of the blister packaging and extract it completely from the packaging.
completely from the packaging.
 Remove the Abfentiq unit from the blister packaging and immediately place the Abfentiq lozenge in your mouth.
Remove the Abfentiq unit from the blister packaging and immediately place the Abfentiq lozenge in your mouth.
Use of the Abfentiq unit
- Place the lozenge between your cheeks and gums.
- With the applicator, continuously move Abfentiq around your mouth, especially your cheeks. Turn the applicator often.
especially your cheeks. Turn the applicator often.
 To make the relief more effective, you must finish the Abfentiq unit completely in about 15 minutes. If you finish it too quickly, you will swallow more medication and get less relief from the breakthrough pain.
To make the relief more effective, you must finish the Abfentiq unit completely in about 15 minutes. If you finish it too quickly, you will swallow more medication and get less relief from the breakthrough pain.
more medication and get less relief from the breakthrough pain.
- Do not bite or chew the Abfentiq unit. This will result in lower blood levels and less relief from the breakthrough pain than if used as indicated.
less relief from the breakthrough pain than if used as indicated.
- If for any reason you do not finish the entire Abfentiq unit each time you experience breakthrough pain, contact your doctor.
Frequency of administration
Once you have achieved a dose that effectively controls your breakthrough pain, do not use more than four units of Abfentiq per day. If you think you may need more than four units of Abfentiq per day, you must notify your doctor immediately.
How many units of Abfentiq should you use
Do not use more than two Abfentiq lozenges to treat a single episode of breakthrough pain.
If you use more Abfentiq than you should
The most common adverse effects if you use too much are drowsiness, dizziness, and nausea.
- If you start to feel dizzy, nauseous, or very sleepy before the lozenge is completely dissolved, remove it from your mouth and ask someone else in the household for help.
someone else in the household for help.
A serious adverse effect of Abfentiq is slow and/or shallow breathing. This can occur if the dose of Abfentiq is too high or if you use too much Abfentiq.
- If this happens, seek medical help immediately.
- In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service. Phone: 91 562 04 20, indicating the medication and the amount ingested.
What to do if a child or adult accidentally uses Abfentiq
If you believe someone has accidentally used Abfentiq, seek medical help immediately. Try to keep the person awake (by calling their name or shaking their arm or shoulder) until medical help arrives.
If you forget to use Abfentiq
If the breakthrough pain still persists, you should use Abfentiq as your doctor has indicated. If the breakthrough pain disappears, do not use more Abfentiq until another episode of breakthrough pain appears.
If you interrupt treatment with Abfentiq
Do not stop using Abfentiq without consulting your doctor. No noticeable effects usually appear if you stop using Abfentiq. Continue using your usual opioid medication to treat persistent pain (present all the time) as your doctor indicates.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, Abfentiq can produce adverse effects, although not all people experience them. If you notice any adverse effect, contact your doctor.
The most serious adverse effects are shallow breathing, low blood pressure, and shock.
You or your caregiver should remove the Abfentiq unit from your mouth. Contact your doctor immediately and request urgent help if you experience any of the following adverse effects – you may need urgent medical attention:
- If you are very drowsy or have slow or shallow breathing.
- Difficulty breathing or feeling dizzy, swelling of the tongue, lips, or throat that can be the first signs of a severe allergic reaction.
Note for caregivers:
If you observe that the patient using Abfentiq has slow and/or shallow breathing or has difficulty waking up, take the following measures IMMEDIATELY:
- Take the Abfentiq unit by the applicator, remove it from the patient's mouth, and keep it out of the reach of children or pets until you dispose of it.
- REQUEST URGENT ASSISTANCE
- While waiting for urgent assistance to arrive, if the person appears to be breathing slowly, encourage them to breathe every 5-10 seconds.
every 5-10 seconds.
If you feel excessively dizzy, drowsy, or experience any other discomfort while using Abfentiq, remove the Abfentiq unit from your mouth using the applicator and dispose of it according to the instructions explained in this prospectus (see section 5). Then, contact your doctor for new instructions on using Abfentiq.
Very common adverse effects(may affect more than 1 in 10 patients):
- Vomiting, nausea/discomfort, constipation, stomach pain (abdominal)
- Asthenia (weakness), drowsiness, sedation, dizziness. Headache.
- Shortness of breath.
Common adverse effects(may affect up to 1 in 10 patients):
- Confusion, anxiety, seeing or hearing things that are not there (hallucinations), depression, mood changes.
- Feeling unwell
- Seizure, muscle spasm, feeling of vertigo or dizziness, loss of consciousness, sedation, feeling of tingling, numbness, difficulty coordinating movements, increased or altered sensitivity to touch, convulsions (epileptic seizures).
- Dry mouth, oral inflammation, tongue disorders (e.g., burning sensation or ulcers), taste alterations.
- Gas, abdominal bloating, indigestion, decreased appetite, weight loss.
- Blurred or double vision.
- Sweating, skin rash, itching.
- Difficulty urinating
- Accidental injuries (e.g., falls).
- General discomfort.
Uncommon adverse effects(may affect up to 1 in 100 patients):
- Dental caries, intestinal paralysis, oral ulcers, gum bleeding.
- Coma, difficulty speaking.
- Abnormal dreams, feeling of indifference, abnormal thoughts, excessive feeling of well-being.
- Vasodilation
- Hives
Frequency not known:
- Decreased gums, gum inflammation, tooth loss, severe respiratory problems, flushing, feeling of excessive heat, diarrhea, inflammation of arms or legs, fatigue.
- Drug dependence (addiction)
- Drug abuse
- Delirium (symptoms may consist of a combination of agitation, restlessness, disorientation, confusion, fear, seeing or hearing things that really do not exist, sleep disorders, nightmares)
While using Abfentiq, you may experience irritation, pain, and ulcers at the application site and gum bleeding.
Prolonged treatment with fentanyl during pregnancy may cause withdrawal symptoms in the newborn, which can be potentially fatal (see section 2).
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Abfentiq
Keep this medication out of sight and reach of children.
The analgesic medication of Abfentiq is very potent and could be potentially fatal for a child if used accidentally. Abfentiq must be kept out of reach and sight of children.
- Do not use this medication after the expiration date that appears on the blister packaging and carton after "EXP:". The expiration date is the last day of the month indicated.
- Store below 25°C.
- Keep Abfentiq always in its blister packaging until you are ready to use it. Do not use this medication if you observe that the blister packaging is damaged or opened before you are ready to use it.
before you are ready to use it.
Medications should not be thrown away in drains or in the trash. Deposit the packaging and medications you no longer need in the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
- How to eliminate Abfentiq once used
Partially used Abfentiq units may still contain enough medication to be harmful or potentially fatal to a child.
Even if there is still medication left in the applicator or not, the applicator must be eliminated properly, as follows:
- If there is no medication left, throw the applicator away in a trash can that is out of the reach of children and pets.
- If there is medication left in the applicator, put the lozenge under a hot water faucet to dissolve the remaining medication and then throw the applicator away in a trash can that is out of the reach of children and pets.
- If you do not finish the entire Abfentiq unit and cannot dissolve the remaining medication immediately, put the Abfentiq unit out of the reach of children and pets until you have time to dispose of the partially used Abfentiq unit as explained.
- Do not throw away partially used Abfentiq units, Abfentiq applicators, or blister packaging down the toilet.
6. Package contents and additional information
Composition of Abfentiq 200, 400, 600, 800, 1200, 1600 micrograms
The active ingredient is fentanyl. Each lozenge contains 200, 400, 600, 800, 1200, 1600 micrograms of fentanyl (as citrate).
The other components are:
Lozenge:
Hydrated dextrates, anhydrous citric acid, anhydrous sodium hydrogen phosphate, artificial berry flavor, magnesium stearate.
Edible adhesive used to attach the lozenge to the applicator:
Modified cornstarch-based edible adhesive (E 1450), hydrated dextrates, water.
Applicator:
ABS resin
Food ink (E-133)
Appearance of the product and package contents
Abfentiq is a system for the administration of medications directly through the oral mucosa. Each Abfentiq unit consists of a solid white medication attached to an applicator.
The unit is normally white; however, during storage, it may acquire a slightly speckled appearance. This is due to slight changes in the product's flavoring and does not affect the medication's action in any way.
Abfentiq exists in 6 different doses: 200, 400, 600, 800, 1200, and 1600 micrograms. The dose is marked on the white lozenge, on the applicator, on the blister packaging, and on the carton, to ensure that you use the correct medication and dose. Each dose is associated with a specific color.
Each blister packaging contains a single Abfentiq unit, supplied in boxes of 3, 6, 15, or 30 individual Abfentiq units.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
FERRER INTERNACIONAL, S.A.
Gran Vía Carlos III, 94
08028 Barcelona (Spain)
Manufacturer:
PRASFARMA, S.L.
c/ Sant Joan 11-15.
08560 Manlleu (Barcelona)
Spain
Local representative:
FERRER FARMA, S.A.
Av. Diagonal, 549 5ª planta
08029 Barcelona (Spain)
Date of the last revision of this prospectus: February 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price206.41 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ABFENTIQ 600 micrograms LOZENGE tabletsDosage form: TRANSDERMAL PATCH, 12 MCG/HActive substance: fentanylManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1200 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1600 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for ABFENTIQ 600 micrograms LOZENGE tablets
Discuss questions about ABFENTIQ 600 micrograms LOZENGE tablets, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















