VICTOZA 6 MG/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar VICTOZA 6 MG/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Victoza 6mg/ml solución inyectable en pluma precargada
liraglutida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Victoza y para qué se utiliza
- Qué necesita saber antes de empezar a usar Victoza
- Cómo usar Victoza
- Posibles efectos adversos
- Conservación de Victoza
- Contenido del envase e información adicional
1. Qué es Victoza y para qué se utiliza
Victoza contiene el principio activo liraglutida. Ayuda a su cuerpo a reducir su nivel de azúcar en sangre únicamente cuando este nivel de azúcar está demasiado elevado. Además, hace más lento el paso de los alimentos por su estómago y puede ayudar a prevenir una enfermedad cardiaca.
Victoza se utiliza él solo si su nivel de azúcar en sangre no está controlado de forma adecuada únicamente con dieta y ejercicio, y no puede utilizar metformina (otro medicamento para la diabetes).
Victoza se utiliza junto con otros medicamentos para la diabetes, cuando estos no son suficientes para controlar su nivel de azúcar en sangre. Estos pueden ser:
- antidiabéticos orales [como metformina, pioglitazona, sulfonilurea, inhibidor del cotransportador sodio-glucosa tipo 2 (iSGLT2)] y/o insulina.
2. Qué necesita saber antes de empezar a usar Victoza
No use Victoza
- si es alérgico a liraglutida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero:
- antes de empezar a usar Victoza.
- si usted tiene o ha tenido una enfermedad del páncreas.
Si sabe que va a someterse a una intervención quirúrgica en la que se someterá a anestesia (estado
de sueño), informe a su médico de que está tomando Victoza.
Este medicamento no se debe utilizar si tiene diabetes tipo 1 (su cuerpo no produce nada de insulina) o cetoacidosis diabética (una complicación de la diabetes que se caracteriza por un alto nivel de azúcar en sangre y un aumento del esfuerzo para respirar). No es una insulina y, por lo tanto, no se debe utilizar como un sustituto de insulina.
No se recomienda el uso de Victoza si está en diálisis.
No se recomienda el uso de Victoza si tiene una enfermedad hepática grave.
El uso de Victoza no está recomendado si padece insuficiencia cardiaca grave.
No se recomienda este medicamento si tiene un problema grave de estómago o de intestino que produce un retraso del vaciado del estómago (llamado gastroparesia), o enfermedad inflamatoria intestinal.
Si presenta síntomas de pancreatitis aguda, como dolor de estómago intenso y continuo, debe consultar a su médico inmediatamente (ver sección 4).
Si padece enfermedad de tiroides, incluyendo nódulos tiroideos y aumento de tamaño de la glándula tiroides, consulte a su médico.
En algunos casos, cuando se inicia un tratamiento con Victoza, se puede experimentar una deshidratación (pérdida de líquidos), por ejemplo, en caso de sufrir vómitos, náuseas y diarrea. Es importante evitar la deshidratación bebiendo mucho líquido. Hable con su médico si tiene alguna duda.
Niños y adolescentes
Se puede utilizar Victoza en adolescentes y niños a partir de 10 años de edad. No hay datos disponibles en niños menores de 10 años de edad.
Otros medicamentos y Victoza
Informe a su médico, farmacéutico o enfermero si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
En concreto, informe a su médico, farmacéutico o enfermero si está utilizando medicamentos que contengan alguno de los siguientes principios activos:
- Sulfonilurea (como glimepirida o glibenclamida) o insulina. Puede sufrir hipoglucemia (nivel de azúcar en sangre bajo) cuando utilice Victoza junto con una sulfonilurea o insulina, ya que las sulfonilureas y la insulina aumentan el riesgo de hipoglucemia. Cuando empiece a utilizar estos medicamentos juntos por primera vez, su médico puede indicarle que reduzca la dosis de sulfonilurea o insulina. Para consultar los síntomas de aviso de una bajada de azúcar en sangre, ver sección 4. Si también está tomando una sulfonilurea (como glimepirida o glibenclamida) o insulina, su médico puede solicitarle un análisis de sus niveles de azúcar en sangre. Esto ayudará a su médico a decidir si es necesario cambiar la dosis de sulfonilurea o insulina.
- Si está recibiendo insulina, su médico le indicará cómo reducir la dosis de insulina y le recomendará que controle su nivel de azúcar en sangre con mayor frecuencia para evitar hiperglucemia (niveles altos de azúcar en sangre) y cetoacidosis diabética (una complicación de la diabetes que se produce cuando el organismo no puede descomponer la glucosa porque no hay suficiente insulina).
- Warfarina u otros medicamentos anticoagulantes. Pueden ser necesarios análisis de sangre más frecuentes para determinar la capacidad de coagulación de su sangre.
Embarazo y lactancia
Informe a su médico si está embarazada, si cree que puede estarlo o si planea estarlo. Victoza no debe utilizarse durante el embarazo porque se desconoce si podría dañar al feto.
Se desconoce si Victoza pasa a la leche materna, por tanto, no utilice este medicamento durante el periodo de lactancia.
Conducción y uso de máquinas
El bajo nivel de azúcar en sangre (hipoglucemia) puede reducir su capacidad de concentración. Evite conducir o usar máquinas si experimenta síntomas de hipoglucemia. Ver sección 4 para consultar los síntomas de aviso de una bajada de azúcar en sangre. Consulte a su médico para más información.
Información importante sobre algunos componentes de Victoza
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Victoza
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
- La dosis inicial es 0,6 mg una vez al día, durante al menos una semana.
- Su médico le indicará cuándo aumentar esa dosis a 1,2 mg una vez al día.
- Su médico puede indicarle que siga aumentando la dosis a 1,8 mg una vez al día, si su glucosa en sangre no se controla adecuadamente con una dosis de 1,2 mg.
No cambie la dosis a menos que su médico se lo indique.
Victoza se administra como una inyección bajo la piel (subcutánea). No la inyecte en una vena o músculo. Las mejores zonas para la inyección son la parte frontal del muslo, la zona del abdomen o la parte superior del brazo. Cambie el lugar dónde se inyecta cada día para reducir el riesgo de desarrollar bultos en la piel.
Se puede administrar la inyección en cualquier momento del día, con independencia de las comidas. Una vez que haya decidido la hora del día más conveniente, es preferible que se inyecte Victoza en torno a la misma hora del día.
Antes de utilizar la pluma por primera vez, su médico o enfermero le mostrarán cómo utilizarla.
En la otra cara de este prospecto encontrará instrucciones detalladas sobre su uso.
Si usa más Victoza del que debe
Si usa más Victoza del que debe, consulte con su médico inmediatamente. Puede que necesite tratamiento médico. Puede que experimente náuseas, vómitos, diarrea o nivel de azúcar en sangre bajo (hipoglucemia). Consulte los síntomas de aviso de una bajada de azúcar en sangre en la sección 4.
Si olvidó usar Victoza
Si olvida una dosis, use Victoza tan pronto como se acuerde.
Sin embargo, si han pasado más de 12 horas desde que debería haber usado Victoza, sáltese la dosis olvidada. Adminístrese la siguiente dosis, al día siguiente, como de costumbre.
No use una dosis doble o aumente la dosis del día siguiente para compensar la dosis olvidada.
Si interrumpe el tratamiento con Victoza
No interrumpa el tratamiento con Victoza sin consultar con su médico. Si lo interrumpe, puede que aumenten sus niveles de azúcar en sangre.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Hipoglucemia (nivel de azúcar en sangre bajo). Los síntomas de aviso de una bajada de azúcar en sangre pueden aparecer repentinamente e incluir: sudor frío, piel fría y pálida, dolor de cabeza, palpitaciones, náuseas, apetito excesivo, trastornos visuales, somnolencia, sensación de debilidad, nerviosismo, ansiedad, confusión, dificultad de concentración y temblores. Su médico le indicará cómo tratar el bajo nivel de azúcar en sangre y qué tiene que hacer en el caso de que observe estos síntomas de aviso. Esto es más probable que suceda si también utiliza una sulfonilurea o insulina. Puede que su médico reduzca su dosis de estos medicamentos antes de que empiece a usar Victoza.
Raros: pueden afectar hasta 1 de cada 1.000 personas
- Una reacción alérgica grave (reacción anafiláctica) con síntomas adicionales tales como problemas respiratorios, hinchazón de la garganta y de la cara, palpitaciones, etc. Si nota alguno de estos síntomas, busque ayuda médica inmediatamente y consulte a su médico tan pronto como sea posible.
- Obstrucción intestinal. Una forma grave de estreñimiento con síntomas adicionales tales como dolor de estómago, hinchazón, vómitos, etc.
Muy raros: pueden afectar hasta 1 de cada 10.000 personas
- Casos de inflamación del páncreas (pancreatitis). La pancreatitis puede ser una enfermedad grave y potencialmente mortal. Deje de usar Victoza y contacte con su médico inmediatamente si usted nota alguno de los siguientes efectos adversos graves:
- dolor intenso y persistente en el abdomen (zona del estómago) que puede llegar hasta la espalda, así como náuseas y vómitos, ya que podría ser un signo de una inflamación del páncreas (pancreatitis).
Otros efectos adversos
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- Náuseas. Este efecto desaparece normalmente con el tiempo.
- Diarrea. Este efecto desaparece normalmente con el tiempo.
Frecuentes
- Vómitos.
Cuando se inicia el tratamiento con Victoza, en algunos casos se puede experimentar pérdida de líquidos/deshidratación. Por ejemplo en caso de vómitos, náuseas y diarrea. Es importante evitar la deshidratación bebiendo mucho líquido.
- Dolor de cabeza
- Indigestión
- Estómago inflamado (gastritis). Los síntomas incluyen dolor de estómago, náuseas y vómitos.
- Enfermedad de reflujo gastroesofágico (ERGE). Los síntomas incluyen pirosis.
- Vientre (abdomen) hinchado o dolor abdominal
- Malestar abdominal
- Estreñimiento
- Gases (flatulencia)
- Disminución del apetito
- Bronquitis
- Resfriado común
- Mareos
- Pulso acelerado
- Cansancio
- Dolor de muelas
- Reacciones en el lugar de la inyección (hematomas, dolor, irritación, picor y sarpullido).
- Aumento de las enzimas pancreáticas (como lipasa y amilasa).
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- Reacciones alérgicas tales como prurito (picor) y urticaria (un tipo de sarpullido cutáneo)
- Deshidratación, a veces con una disminución de la función renal
- Malestar (no sentirse bien)
- Piedras en la vesícula
- Vesícula biliar inflamada
- Cambio en el sabor de las cosas
- Retraso en el vaciamiento gástrico.
Frecuencia no conocida: no se puede estimar la frecuencia a partir de los datos disponibles
- Los bultos bajo la piel pueden producirse por la acumulación de una proteína denominada amiloide (amiloidosis cutánea; no se conoce la frecuencia con la que esto ocurre).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Victoza
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el envase de la pluma después de CAD. La fecha de caducidad es el último día del mes que se indica.
Antes de usar:
Conservar en nevera (entre 2 °C y 8 °C). No congelar. Mantener alejado del congelador.
Durante el uso:
Puede conservar la pluma durante un mes si se almacena por debajo de 30 °C o en nevera (entre 2 °C y 8 °C), alejado del congelador. No congelar.
Cuando no se utilice, conservar la pluma con el capuchón puesto para protegerla de la luz.
No utilice este medicamento si observa que la solución no es transparente e incolora o casi incolora.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Victoza
- El principio activo es liraglutida. 1 ml de solución inyectable contiene 6 mg de liraglutida. Una pluma precargada contiene 18 mg de liraglutida.
- Los demás componentes son fosfato disódico dihidrato, propilenglicol, fenol y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Victoza se suministra como una solución inyectable transparente e incolora o casi incolora en una pluma precargada. Cada pluma contiene 3 ml de solución, pudiendo suministrar 30 dosis de 0,6 mg, 15 dosis de 1,2 mg o 10 dosis de 1,8 mg.
Victoza está disponible en envases de 1, 2, 3, 5 o 10 plumas. Puede que solamente estén comercializados algunos tamaños de envases.
Las agujas no están incluidas.
Titular de la autorización de comercialización y responsable de la fabricación
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Dinamarca
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
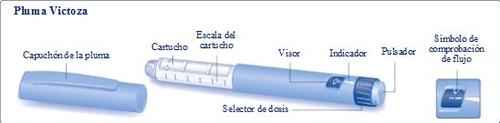
INSTRUCCIONES DE USO DE LA PLUMA VICTOZA Lea detenidamente estas instrucciones antes de utilizar su pluma. Su pluma contiene 18 mg de liraglutida. Puede seleccionar dosis de 0,6 mg, 1,2 mg y 1,8 mg. La pluma está diseñada para ser utilizada con agujas de inyección desechables NovoFine o NovoTwist de una longitud de hasta 8 mm y tan fina como de un calibre de hasta 32 G (0,25/0,23 mm). |
|
| |
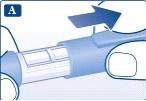
Preparación de la pluma Compruebe el nombre y el color de la etiquetade su pluma para asegurarse de que contiene liraglutida. El uso de un medicamento incorrecto podría producirle graves daños. Retire el capuchón de la pluma. |
|
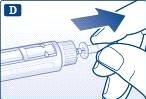
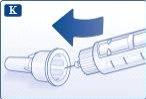
Retire la lengüeta de papel de una nueva aguja desechable. Enrosque recta y firmemente la aguja en la pluma. |
|
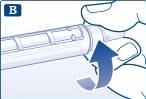
Retire el capuchón exterior de la aguja y guárdelo para más tarde. |
|
Retire el capuchón interior de la aguja y deséchelo. |
|
Utilice siempre una aguja nueva para cada inyección. Así se reduce el riesgo de contaminación, infección, pérdida de liraglutida, que las agujas se atasquen y las dosificaciones inexactas. Tenga cuidado de no doblar o dañar la aguja. No intente volver a poner nunca el capuchón interior de la aguja. Podría pincharse con la aguja. | |
Mantenimiento de su pluma
| |
Información importante
| |
Con cada nueva pluma, compruebe el flujo Compruebe el flujo antes de la primera inyección con cada pluma nueva. Si su pluma está ya en uso, vaya a “Selección de la dosis”, paso H. Gire el selector de dosis hasta que el indicador señale el símbolo de comprobación de flujo. |
|
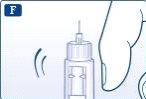
Sujete la pluma con la aguja apuntando hacia arriba. Golpee el cartucho suavemente con el dedo varias veces. De este modo las burbujas de aire se concentran en la parte superior del cartucho. |
|
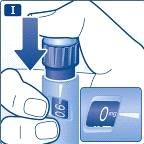
Mantenga la aguja apuntando hacia arriba y pulse el pulsador hasta que el indicador señale 0 mg. Una gota de liraglutida debe aparecer en la punta de la aguja. Si no aparece ninguna gota, repita los pasos Ea Ghasta cuatro veces. Si aún no se ve ninguna gota de liraglutida, cambie la aguja y repita los pasos Ea Guna vez más. No use la pluma si todavía no ha aparecido una gota de liraglutida. Esto indica que la pluma está defectuosa y debe utilizar una nueva. |
|
Si se le ha caído la pluma en una superficie dura o sospecha que no funciona correctamente, coloque siempre una nueva aguja desechable y compruebe el flujo antes de utilizarla. | |
Selección de la dosis Compruebe siempre que el indicador señala 0mg. Gire el selector de dosis hasta que el indicador señale la dosis necesaria (0,6 mg, 1,2 mg o 1,8 mg). Si selecciona por error una dosis equivocada, para cambiarla solo hay que girar el selector de dosis hacia atrás o hacia delante hasta que el indicador señale la dosis correcta. Tenga cuidado de no presionar el pulsador de inyección mientras gira el selector de dosis hacia atrás, ya que puede salir liraglutida. Si el selector de dosis se detiene antes de que el indicador señale la dosis necesaria, significa que no queda suficiente liraglutida para una dosis completa. En ese caso puede: Dividir su dosis en dos inyecciones: Gire el selector de dosis en cualquier dirección hasta que el indicador señale 0,6 mg o 1,2 mg. Inyecte la dosis. Después prepare una pluma nueva e inyéctese el número restante de mg para completar su dosis. Solo debe dividir su dosis entre la pluma actual y una pluma nueva si ha recibido la formación adecuada o los consejos de su profesional sanitario. Utilice una calculadora para planificar las dosis. Si divide la dosis de forma equivocada, puede inyectarse demasiada o muy poca cantidad de liraglutida. Inyecte la dosis completa con una pluma nueva: Si el selector de dosis se detiene antes de que el indicador señale 0,6 mg, prepare una pluma nueva e inyéctese con ella la dosis completa. |
|
No intente seleccionar otras dosis que no sean 0,6 mg, 1,2 mg o 1,8 mg. Los números del visor tienen que estar alineados de manera exacta con el indicador para asegurar que recibe la dosis correcta. Oirá un clic cada vez que gire el selector de dosis. No utilice estos clics para seleccionar su dosis. No utilice la escala del cartucho para medir la cantidad de liraglutida que se va a inyectar ya que no es lo bastante preciso. | |
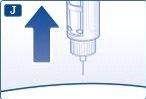
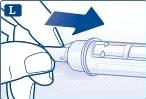
Inyección de la dosis Introduzca la aguja bajo la piel según la técnica de inyección aconsejada por su médico o enfermero.A continuación, siga estas instrucciones: Presione el pulsador hasta que el indicador señale 0 mg. Tenga cuidado de no tocar el visor con los otros dedos ni de pulsar el selector de dosis de lado mientras se inyecta. El motivo es que se puede bloquear la inyección. Mantenga presionado el pulsador y deje la aguja bajo la piel durante al menos 6 segundos. Esto garantiza que se administra la dosis completa. |
|
Retire la aguja. A continuación, puede que vea una gota de liraglutida en la punta de la aguja. Esto es normal y no afecta a su dosis. |
|
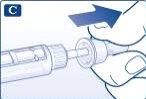
Inserte la punta de la aguja en el capuchón exterior de la aguja sin tocar la aguja o el capuchón exterior de la aguja. |
|
Cuando la aguja quede protegida, presione con cuidado el capuchón exterior de la aguja hasta el fondo. A continuación, desenrosque la aguja. Deseche con cuidado la aguja y vuelva a colocar el capuchón de la pluma. Cuando la pluma esté vacía, deséchela con cuidado sin ninguna aguja puesta. Por favor deseche la pluma y la aguja de acuerdo a las normativas locales. |
|
Retire siempre la aguja después de cada inyección, y guarde su pluma sin la aguja puesta. Así se reduce el riesgo de contaminación, infección, pérdida de liraglutida, que las agujas se atasquen y las dosificaciones inexactas. Las personas que atienden a los pacientes deben tener mucho cuidado cuando manejen agujas usadas para evitar pinchazos accidentales e infecciones. |
- País de registro
- Precio medio en farmacia114.72 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VICTOZA 6 MG/ML SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 6 mg/mlPrincipio activo: LiraglutidaFabricante: Sun Pharmaceutical Industries (Europe) B.V.Requiere recetaForma farmacéutica: INYECTABLE, 6 mg/mlPrincipio activo: LiraglutidaFabricante: Sun Pharmaceutical Industries (Europe) B.V.Requiere recetaForma farmacéutica: INYECTABLE, 6 mg/mlPrincipio activo: LiraglutidaFabricante: Zentiva K.S.Requiere receta
Médicos online para VICTOZA 6 MG/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VICTOZA 6 MG/ML SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes