
SMOFKABIVEN LOW OSMO PERIFERICO EMULSION PARA PERFUSION

Cómo usar SMOFKABIVEN LOW OSMO PERIFERICO EMULSION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es SmofKabiven Low Osmo Periférico y para qué se utiliza
- Qué necesita saber antes de empezar a usar SmofKabiven Low Osmo Periférico
- Cómo usar SmofKabiven Low Osmo Periférico
- Posibles efectos adversos
- Conservación de SmofKabiven Low Osmo Periférico
- Contenido del envase e información adicional
Introducción
Prospecto: Información para el usuario
SmofKabiven Low Osmo Periférico emulsión para perfusión
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es SmofKabiven Low Osmo Periférico y para qué se utiliza
- Qué necesita saber antes de empezar a usar SmofKabiven Low Osmo Periférico
- Cómo usar SmofKabiven Low Osmo Periférico
- Posibles efectos adversos
- Conservación de SmofKabiven Low Osmo Periférico
- Contenido del envase e información adicional
1. Qué es SmofKabiven Low Osmo Periférico y para qué se utiliza
SmofKabiven Low Osmo Periférico es una emulsión para perfusión que se administra en su sangre mediante un gotero (perfusión intravenosa). El producto contiene aminoácidos (componentes utilizados en la formación de proteínas), glucosa (carbohidratos), lípidos (grasa) y sales (electrolitos), en una bolsa de plástico y puede ser administrado a adultos y niños a partir de 2 años de edad.
Un profesional sanitario le administrará SmofKabiven Low Osmo Periférico cuando otras formas de alimentación no sean suficientemente buenas o no sean posibles.
2. Qué necesita saber antes de empezar a usar SmofKabiven Low Osmo Periférico
No use SmofKabiven Low Osmo Periférico:
- si es alérgico (hipersensible) a los principios activos o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si usted es alérgico al pescado o al huevo
- si usted es alérgico a los cacahuetes o a la soja, no debería utilizar este producto. SmofKabiven Low Osmo Periférico contiene aceite de soja
- si usted tiene demasiados lípidos en su sangre (hiperlipidemia)
- si usted padece una alteración hepática grave
- si usted sufre problemas de coagulación de la sangre (alteraciones de la coagulación)
- si su organismo presenta problemas para la utilización de los aminoácidos
- si usted sufre enfermedad renal grave sin posibilidad de diálisis
- si usted se encuentra en shock agudo
- si usted tiene demasiado azúcar en su sangre (hiperglucemia), que no está controlada
- si usted tiene niveles elevados en sangre (suero) de las sales (electrolitos) incluidas en SmofKabiven Low Osmo Periférico
- si usted tiene líquido en los pulmones (edema pulmonar agudo)
- si usted tiene demasiado líquido en su organismo (hiperhidratado)
- si usted presenta insuficiencia cardíaca que no está en tratamiento
- si usted tiene un defecto en su sistema de coagulación de la sangre (síndrome hemofagocitótico)
- si usted se encuentra en una situación inestable, como después de un trauma grave, diabetes mellitus no controlada, ataque cardíaco agudo, derrame cerebral, coágulo de sangre, acidosis metabólica (una alteración que da lugar a demasiado ácido en su sangre), infección grave (sepsis grave), coma, y si usted no tiene suficiente líquido en su organismo (deshidratación hipotónica).
- en niños recién nacidos o menores de 2 años
Advertencias y precauciones
Consulte a su médico antes de empezar a usar SmofKabiven Low Osmo Periférico si tiene:
- problemas renales
- diabetes mellitus
- pancreatitis (inflamación del páncreas)
- problemas hepáticos
- hipotiroidismo (problemas tiroideos)
- sepsis (infección grave)
Si durante la perfusión aparece fiebre, erupción cutánea, hinchazón, dificultad para respirar, escalofríos, sudoración, náuseas o vómitos, informe a su profesional sanitario inmediatamente, porque estos síntomas podrían ser causados por una reacción alérgica, o porque usted está recibiendo demasiada cantidad del medicamento.
Su doctor necesitará controlar regularmente su sangre, por medio de análisis de la función hepática y otros valores.
Niños y adolescentes
SmofKabiven Low Osmo Periférico no está pensado para niños recién nacidos ni niños de menos de 2 años de edad. SmofKabiven Low Osmo Periférico puede ser administrado a niños de 2 a 18 años de edad.
Uso de SmofKabiven Low Osmo Periférico con otros medicamentos
Informe a su médico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento incluso los adquiridos sin receta.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
No existe información sobre el uso de SmofKabiven Low Osmo Periférico durante el embarazo. SmofKabiven Low Osmo Periférico debería administrarse a mujeres embarazadas únicamente si el médico lo considera absolutamente necesario. Puede considerarse el uso de SmofKabiven Low Osmo Periférico en el embarazo, si su médico lo aconseja.
No hay datos disponibles sobre la exposición en mujeres en periodo de lactancia.
Los componentes y metabolitos de la nutrición parenteral como SmofKabiven Low Osmo Periférico se excretan en la leche humana. La nutrición parenteral puede ser necesaria durante la lactancia. SmofKabiven Low Osmo Periférico solo debe administrarse a mujeres en periodo de lactancia cuando el médico haya sopesado los potenciales riesgos y beneficios.
Conducción y uso de máquinas
No es relevante, ya que este medicamento se administra en el hospital.
3. Cómo usar SmofKabiven Low Osmo Periférico
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Su médico decidirá la dosis para usted de forma individual dependiendo de su peso corporal y su situación. SmofKabiven Low Osmo Periférico le será administrado por un profesional sanitario.
Si usa más SmofKabiven Low Osmo Periférico del que debe
Es muy poco probable que usted reciba demasiada cantidad de medicamento, ya que SmofKabiven Low Osmo Periférico le será administrado por un profesional sanitario.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuentes(pueden afectar hasta 1 de cada 10 pacientes): un ligero aumento de la temperatura corporal, inflamación en venas periféricas superficiales en conexión con el sitio de inyección.
Poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes): niveles elevados en sangre (plasma) de componentes hepáticos, ausencia de apetito, náuseas, vómitos, escalofríos, mareos y dolor de cabeza.
Raros(pueden afectar hasta 1 de cada 1000 pacientes): presión sanguínea baja o elevada, dificultad para respirar, frecuencia cardíaca rápida (taquicardia). Reacciones de hipersensibilidad (que pueden dar síntomas como hinchazón, fiebre, descenso de la presión sanguínea, erupciones cutáneas, ronchas (zonas rojas hinchadas), enrojecimiento, dolor de cabeza). Sensaciones de frío y calor. Palidez. Labios y piel con coloración azulada (debido a la falta de oxígeno en su sangre). Dolor en cuello, espalda, huesos, pecho y zona lumbar.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de SmofKabiven Low Osmo Periférico
Mantener este medicamento fuera de la vista y del alcance de los niños.
Mantener en la sobrebolsa. No conservar a temperatura superior a 25º C. No congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la bolsa y de la caja. La fecha de caducidad es el último día del mes que se indica.
6. Contenido del envase e información adicional
SmofKabiven Low Osmo Periférico contiene
Las sustancias activas son | g por 1.000 ml |
Glucosa (como monohidrato) | 68 |
Alanina | 3,5 |
Arginina | 3,0 |
Glicina | 2,8 |
Histidina | 0,75 |
Isoleucina | 1,3 |
Leucina | 1,9 |
Lisina (como acetato) | 1,7 |
Metionina | 1,1 |
Fenilalanina | 1,3 |
Prolina | 2,8 |
Serina | 1,6 |
Taurina | 0,25 |
Treonina | 1,1 |
Triptófano | 0,50 |
Tirosina | 0,10 |
Valina | 1,6 |
Cloruro cálcico (como dihidrato) | 0,14 |
Glicerofosfato sódico (como hidrato) | 1,0 |
Sulfato magnésico (como heptahidrato) | 0,30 |
Cloruro potásico | 1,1 |
Acetato sódico (como trihidrato) | 0,85 |
Sulfato de zinc (como heptahidrato) | 0,0032 |
Aceite de soja, refinado | 11 |
Triglicéridos de cadena media | 11 |
Aceite de oliva, refinado | 8,8 |
Aceite de pescado, rico en ácidos grasos omega-3 | 5,3 |
Los demás componentes son: glicerol, fosfolípidos de huevo purificados, todo-rac-α-tocoferol, hidróxido sódico (ajuste pH), oleato sódico, ácido acético glacial (ajuste pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Las soluciones de glucosa y aminoácidos son transparentes, incoloras o ligeramente amarillas y libres de partículas. La emulsión lipídica es blanca y homogénea.
Tamaños de envase:
1 x 850 ml, 5 x 850 ml
1 x 1.400 ml, 4 x 1.400 ml
1 x 1.950 ml, 4 x 1.950 ml
1 x 2.500 ml, 3 x 2.500 ml
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Fresenius Kabi España S.A.U.
C/ Marina 16-18.
08005 Barcelona (España )
Responsable de la fabricación
Fresenius Kabi AB, SE-751 74 Uppsala, Suecia
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Nombre del estado miembro | Nombre del medicamento |
Austria | SmofKabiven Low Osmo peripher Emulsion zur Infusion |
Belgium | SmofKabiven Low Osmo Perifeer Smofkabiven Low Osmo Périphérique SmofKabiven Low Osmo Peripher |
Bulgaria | ??????????? ??? ???? ????????? ?????????? ??????? |
Croatia | SmofKabiven Low Osmo Peripheral |
Cyprus | SmofKabiven Low Osmo Peripheral |
Czech Rep. | SmofKabiven Low Osmo Peripheral |
Denmark | SmofKabiven Low Osmo Peripheral |
Estonia | SmofKabiven Low Osmo Peripheral |
Finland | SmofKabiven Low Osmo Peripheral |
Germany | SmofKabiven Low Osmo peripher Emulsion zur Infusion |
Greece | SmofKabiven Low Osmo Peripheral |
Hungary | SmofKabiven Low Osmo Peripheral |
Iceland | SmofKabiven Low Osmo Peripheral |
Ireland | SmofKabiven Low Osmo Peripheral |
Latvia | SmofKabiven Low Osmo Peripheral |
Lithuania | SmofKabiven Low Osmo Peripheral |
Luxembourg | SmofKabiven Low Osmo peripher Emulsion zur Infusion |
Netherlands | SmofKabiven Low Osmo Perifeer |
Norway | SmofKabiven Low Osmo Peripheral |
Poland | SmofKabiven Low Osmo Peripheral |
Portugal | SmofKabiven Low Osmo Peripheral |
Romania | SmofKabiven Low Osmo Peripheral emulsie perfuzabila |
Slovakia | SmofKabiven Low Osmo Peripheral |
Slovenia | SmofKabiven Peripheral Low Osmo emulzija za infundiranje |
Spain | SmofKabiven Low Osmo Periférico. |
Sweden | SmofKabiven Low Osmo Peripheral |
United Kingdom | SmofKabiven Low Osmo Peripheral |
Fecha de la última revisión de este prospecto: Julio 2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Advertencias y precauciones especiales de uso
Para evitar los riesgos asociados con velocidades de perfusión demasiado rápidas, se recomienda el uso de una perfusión continua y bien controlada, si es posible mediante el uso de una bomba de perfusión.
Dado que el uso de una vena periférica está asociado a un elevado riesgo de infección, deben tomarse precauciones asépticas estrictas para evitar cualquier contaminación durante la inserción del catéter y la manipulación.
Deben monitorizarse la glucosa sérica, los electrolitos y la osmolaridad, así como el balance hídrico, el equilibrio ácido-base y los niveles de enzimas hepáticos.
Ante cualquier signo o síntoma de reacción anafiláctica (como fiebre, temblores, erupción cutánea o disnea) debe interrumpirse inmediatamente la perfusión.
SmofKabiven Low Osmo Periférico no debería ser administrado simultáneamente con sangre en el mismo equipo de perfusión, debido al riesgo de pseudoaglutinación.
Se puede producir tromboflebitis si se usan venas periféricas para la perfusión. Debe vigilarse diariamente el sitio de inserción del catéter para detectar signos locales de tromboflebitis.
Forma de administración
Vía intravenosa, perfusión en una vena periférica o central.
Para proporcionar una nutrición parenteral completa, deben añadirse a SmofKabiven Low Osmo Periférico oligoelementos, vitaminas y posiblemente electrolitos (teniendo en cuenta los electrolitos ya presentes en SmofKabiven Low Osmo Periférico), de acuerdo con las necesidades del paciente.
Posología
Adultos
Dosificación:
El rango de dosis de 20 ml - 40 ml de SmofKabiven Low Osmo Periférico /kg pc/día corresponden a 0,08-0,16 g nitrógeno/kg pc/día (0,5-1,0 g de aminoácidos/kg pc/día) y 14-29 kcal/kg pc/día de energía total (12-25 kcal/kg pc/día de energía no-proteica).
Velocidad de perfusión
La velocidad de perfusión máxima para glucosa es 0,25 g/kg pc/h, para los aminoácidos 0,1 g/kg pc/h, y para lípidos 0,15 g/kg pc/h.
La velocidad de perfusión no debe exceder de 3,7 ml/kg pc/hora (correspondiente a 0,25 g de glucosa, 0,09 g de aminoácidos, y 0,13 g de lípidos/kg pc/h). El período de perfusión recomendado es de 12-24 horas.
Dosis máxima diaria
La dosis máxima diaria varía con la situación clínica del paciente e incluso puede cambiar de un día a otro. La dosis diaria máxima recomendada es de 40 ml/kg pc/día.
Población pediátrica
Niños (2-11 años)
Dosificación:
La dosis de hasta 40 ml/kg pc/día debe ser ajustada regularmente de acuerdo con los requerimientos del paciente pediátrico que varían más que en los pacientes adultos.
Velocidad de perfusión:
La velocidad máxima de perfusión recomendada es de 4,0 ml/kg pc/h (correspondiente a 0,10 g de aminoácidos /kg/h, 0,27 g/glucosa/kg/h y 0,14 g lípidos/kg/h). A la velocidad de perfusión máxima recomendada, no usar periodos de perfusión mayores de 10 horas , excepto en casos excepcionales y bajo una estrecha monitorización.
El periodo de perfusión recomendado es de 12-24 horas.
Dosis máxima diaria:
La dosis máxima diaria varía con la condición clínica del paciente y puede incluso cambiar de día a día. La dosis máxima diaria es de 40 ml/kg pc/día.
Adolescentes (12-18 años)
SmofKabiven Low Osmo Periférico puede usarse en adolescentes de la misma forma que en los adultos.
Precauciones de eliminación
No utilizar si el envase está deteriorado.
Utilizar sólo si las soluciones de aminoácidos y glucosa son transparentes e incoloras o ligeramente amarillas, y si la emulsión lipídica es blanca y homogénea. Debe mezclarse el contenido de las tres cámaras separadas antes de utilizar, y antes de realizar cualquier adición a través del puerto de aditivos. Después de la apertura de las soldaduras tipo peel, la bolsa debe ser invertida varias veces con el fin de garantizar una mezcla homogénea, que no muestre evidencia de una separación de fases.
Para un solo uso. Debe desecharse cualquier mezcla sobrante después de la perfusión.
Compatibilidad
Están disponibles datos de compatibilidad con los productos de marca Dipeptiven, Supliven, Vitalipid Adultos, Soluvit (liofilizado) y Glycophos en cantidades definidas y soluciones genéricas de sodio o potasio en concentraciones definidas. Al adicionar los sodio, potasio o fosfato, deben tenerse en cuenta las cantidades ya presentes en la bolsa de acuerdo con las necesidades clínicas del paciente. Los datos generados respaldan adiciones a la bolsa activada de acuerdo con la tabla siguiente:
Volumen | |
SmofKabiven Low Osmo Periférico | 850 ml, 1.400 ml, 1.950 ml y 2.500 ml |
Aditivo | |
Dipeptiven | 0 - 300 ml |
Supliven | 0 - 10 ml |
Soluvit (liofilizado) | 0 - 1 vial |
Vitalipid Adultos | 0 - 10 ml |
Intervalo de electrolito* | |
Sodio | ≤ 150 mmol/l |
Potasio | ≤ 150 mmol/l |
Fosfato (Glycophos) | ≤ 15 mmol/l |
- Incluyendo las cantidades presentes en la bolsa
Nota: Esta tabla indica la compatibilidad. No es una pauta de dosificación.
Las adiciones deben realizarse asépticamente.
Período de validez después de la mezcla
Se ha demostrado la estabilidad física y química de la bolsa de tres cámaras mezclada durante 36 horas a 25°C. Desde un punto de vista microbiológico, el producto debería utilizarse inmediatamente. Si no es utilizado inmediatamente, el tiempo de conservación hasta su utilización y las condiciones previas a su uso son responsabilidad del usuario y normalmente no deberían ser superiores a 24 horas a 2-8º C.
Período de validez después de la mezcla con aditivos
Desde un punto de vista microbiológico, el producto debería utilizarse inmediatamente después de realizar las adiciones. Si no es utilizado inmediatamente, el tiempo de conservación hasta su utilización y las condiciones previas a su uso son responsabilidad del usuario. El tiempo de conservación normalmente no debería ser superior a 24 horas a 2-8º C.
Instrucciones para el uso de SmofKabiven Low Osmo Periférico
La bolsa
850 ml, 1.400 ml, 1.950 ml, 2.500ml
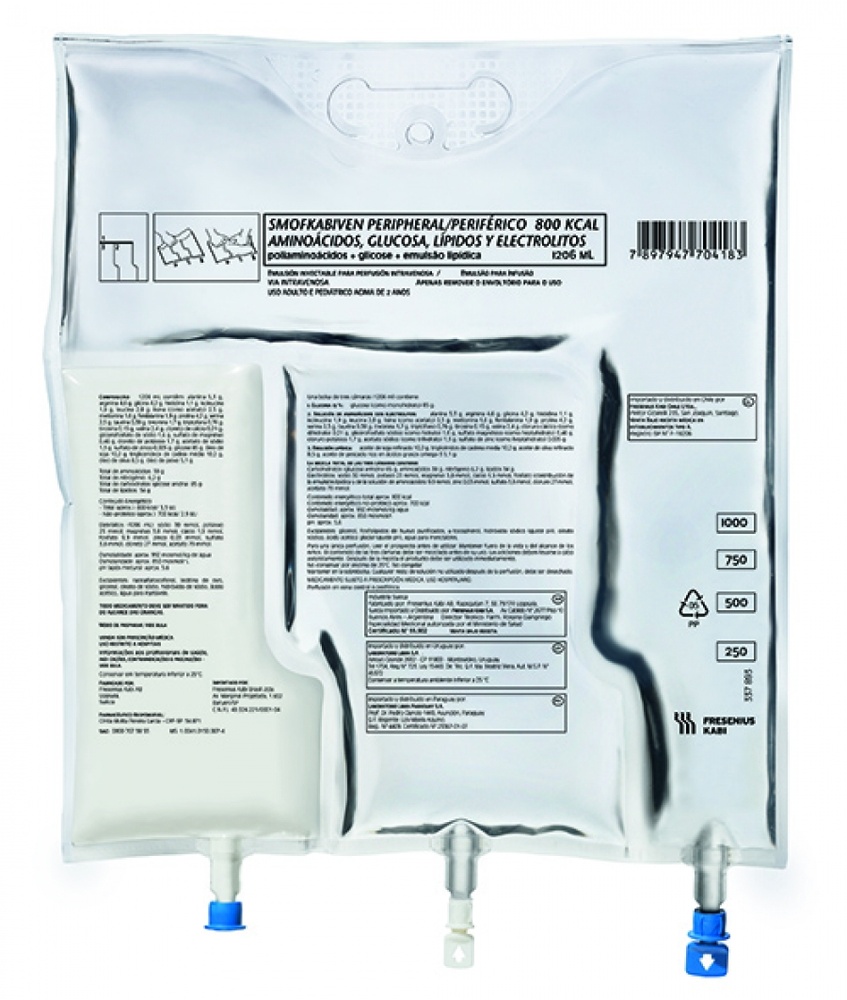
- Muescas en la sobrebolsa
- Asa
- Orificio para colgar la bolsa
- Precintos rompibles
- Puerto ciego (utilizado sólo durante la fabricación)
- Puerto de adición
- Puerto de perfusión
- Absorbente de oxígeno
- Apertura de la sobrebolsa
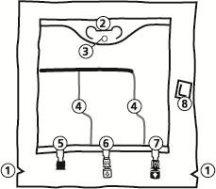
- Para extraer la sobrebolsa, sujetarla en posición horizontal y rasgar por la muesca hacia los puertos a lo largo del borde superior (A)
- Después, simplemente rasgar a lo largo del envase; separar la sobrebolsa y desecharla junto con el absorbente de oxígeno (B).
- Mezclado
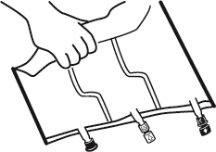
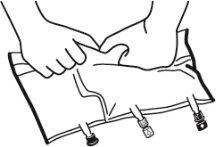
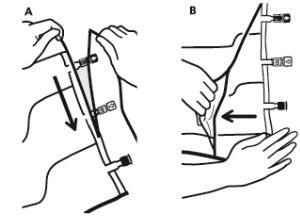
- Colocar la bolsa en una superficie plana.
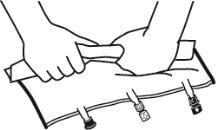
- Enrollar la bolsa desde la parte del colgador hacia la parte de los puertos, primero con la mano derecha y a continuación aplicando una presión constante con la mano izquierda hasta que las soldaduras verticales se hayan abierto. Las soldaduras peel verticales se abren debido a la presión del líquido. Las soldaduras peel también pueden abrirse antes de retirar la sobrebolsa.
Nota:los líquidos se mezclan fácilmente aunque la soldadura horizontal permanezca cerrada.
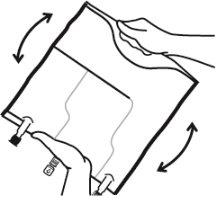
- Mezclar los contenidos de las tres cámaras invirtiendo la bolsa tres veces hasta que los componentes estén completamente mezclados.
- Finalización de la preparación:
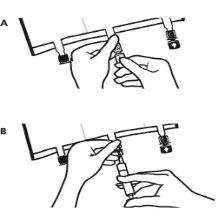
- Colocar la bolsa de nuevo sobre una superficie plana. Poco antes de inyectar los aditivos, romper el puerto de adición blanco por la marca en forma de flecha (A).
Nota:La membrana del puerto de aditivos es estéril.
- Sujetar la base del puerto de aditivos. Insertar la aguja, inyectar los aditivos (de compatibilidad conocida) por el centro del punto de inyección (B).
- Mezclar completamente entre cada adición, invirtiendo la bolsa tres veces. Utilizar jeringas con agujas de calibre 18-23 y una longitud máxima de 40 mm.
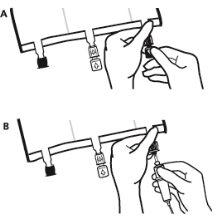
- Poco antes de insertar el set de perfusión, romper el puerto de perfusión azul por la marca en forma de flecha (A).
Nota:La membrana del puerto de perfusión es estéril.
- Usar un equipo de perfusión no venteado o cerrar la entrada del aire del equipo venteado.
- Sujetar la base del puerto de perfusión.
- Introducir el punzón a través del puerto de perfusión. El punzón deberá estar totalmente insertado para asegurar su retención.
Nota:La parte interna del puerto de perfusión es estéril.
- Colgado de la bolsa
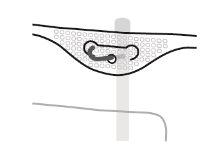
- Colgar la bolsa por la anilla que hay bajo el colgador
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SMOFKABIVEN LOW OSMO PERIFERICO EMULSION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 4,76 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 g / 3,5 g / 200 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 3,5 g / 200 g / 5,22 g / 1,88 g / 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 662 mg / 1,02 g / 4,76 g / 5,15 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 4,25 g / 300 g / 5,22 g / 1,54 g / 4,76 g / 1,53 g / 8,76 g / 4,08 g / 5,1 g / 6,2 g / 3,57 g / 3,4 g / 662 mg / 1,02 g / 5,78 g / 5,94 g / 6,16 g / 4,93 g / 17,6 g / 0,34 g / 9,78 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere receta
Médicos online para SMOFKABIVEN LOW OSMO PERIFERICO EMULSION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SMOFKABIVEN LOW OSMO PERIFERICO EMULSION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















