
SATIVEX 2.7 mg/2.5 mg ORAL BUCCAL SPRAY SOLUTION

How to use SATIVEX 2.7 mg/2.5 mg ORAL BUCCAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Sativex 2.7 mg / 2.5 mg Oral Spray Solution
Delta-9-tetrahydrocannabinol/cannabidiol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Sativex and what is it used for
- What you need to know before you use Sativex
- How to use Sativex
- Possible side effects
- Storing Sativex
- Contents of the pack and other information
1. What is Sativex and what is it used for
What is Sativex
Sativex is an oral spray solution that contains cannabis extracts (called “cannabinoids”).
What is Sativex used for
Sativex is used in multiple sclerosis (MS) to improve symptoms related to muscle stiffness, also called “spasticity”.
The term “spasticity” means that there is an increase in “muscle tone” that makes the muscles feel stiffer or more rigid. This means more difficulty than usual in moving the muscles.
Sativex is used when other medicines have not helped to improve muscle stiffness.
4-week trial period with Sativex
Only a specialist doctor can tell you to start treatment with Sativex.
- Before you start using Sativex, your specialist doctor will do a complete assessment of the severity of your spasticity to assess the degree of muscle stiffness and your response to other treatments.
- Then you will be put on a 4-week trial period with Sativex. After this, your specialist doctor will do another assessment to determine if Sativex is helping you.
- Only if you show significant improvement in symptoms related to spasticity after these 4 weeks can you continue to receive Sativex.
2. What you need to know before you use Sativex
Do not use Sativex:
- If you are allergic to cannabis extracts or any of the other ingredients of this medicine (listed in Section 6).
- If you or someone closely related to you has a history of mental disorder, such as schizophrenia, psychosis, or other major psychiatric disorder. This does not include depression due to your multiple sclerosis.
- If you are breastfeeding.
Do not use this medicine if you have any of the above circumstances. If in doubt, consult your doctor or pharmacist before using Sativex.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Sativex:
- If you are pregnant or trying to become pregnant.
- If you are under 18 years of age.
- If you have epilepsy or frequent epileptic seizures (convulsions).
- If you have kidney problems.
- If you have moderate or severe liver problems.
- If you have a serious heart problem, such as angina, previous heart attack, poorly controlled high blood pressure, or any disorder related to heart rate or rhythm.
- If you are elderly, especially if you have problems performing daily activities such as preparing hot food and drinks.
- If you have a history of drug abuse or substance abuse.
Both you and your partner should use reliable contraceptive measures during the use of this medicine (see the section “Pregnancy, breastfeeding, and contraception (men and women)” below).
If you have any of the above circumstances (or are unsure), consult your doctor or pharmacist before using Sativex.
Using Sativex with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, as Sativex may interfere with the action of some medicines. Similarly, some medicines may interfere with the action of Sativex.
In particular, tell your doctor or pharmacist if you are using medicines for:
- anxiety or insomnia (sedatives or hypnotics such as benzodiazepines, e.g., diazepam or triazolam; other sedatives, e.g., zopiclone, zolpidem, buspirone, St. John's Wort (a herbal preparation))
- muscle spasms (such as baclofen)
- bacterial infections (antibiotics such as rifampicin, clarithromycin)
- epilepsy or nerve pain (such as phenytoin, phenobarbital, carbamazepine)
- high cholesterol (known as statins; e.g., atorvastatin or simvastatin)
- fungal infections (such as itraconazole, fluconazole, and ketoconazole)
- HIV infection (e.g., ritonavir)
- blood thinning (known as coumarins; e.g., warfarin)
- heart problems (known as beta-blockers; e.g., bisoprolol, propranolol)
- corticosteroids for inflammation (such as hydrocortisone, beclometasone, prednisolone)
- certain hormonal medicines used for contraception or for some types of cancer (such as ethinylestradiol, levonorgestrel, or dydrogesterone)
- anesthesia to put you to sleep and relax your muscles before surgery (such as propofol)
If you have any of the above circumstances (or are unsure), consult your doctor or pharmacist before using Sativex.
If you go to see another doctor or go to hospital, tell them what medicines you are using.
Using Sativex with food, drinks, and alcohol
- Generally, you should avoid drinking alcoholic beverages during the use of Sativex, especially at the start of treatment or when changing the dose. If you drink alcoholic beverages while using Sativex, keep in mind that taking alcohol and Sativex together may increase the effects of both (causing loss of balance or altered response), which could increase the risk of falls and other accidents.
- You can use Sativex with or without food (see the section “How to use Sativex”).
Pregnancy, breastfeeding, and contraception (men and women)
- If you are pregnant, think you may be pregnant, or are planning to have a baby, consult your doctor or pharmacist before using this medicine.
Do not use Sativex during pregnancy, unless your doctor tells you to. Sativex may affect the effectiveness of contraceptive methods, such as the “pill” or contraceptive implants. This means you should use an additional contraceptive method.
- Both you and your partner should use a reliable barrier method of contraception, such as a condom, diaphragm, or cervical cap, while using this medicine and for at least 3 months after stopping treatment.
- Do not use Sativex during breastfeeding.
Driving and using machines
- Do not drive or operate machinery when you first start taking Sativex or until you are taking a stable daily dose.
- Sativex may cause drowsiness or dizziness, which may affect your judgment and ability to perform tasks that require skill. On rare occasions, it may cause a brief loss of consciousness.
- Even if you are used to taking Sativex and your dose is stable, do not drive or operate machinery if Sativex causes effects such as drowsiness or dizziness that may affect your ability to perform these tasks. If in doubt, do not drive or operate machinery.
Consult your doctor or pharmacist if you are unsure whether it is safe to drive while taking this medicine.
Traveling abroad with Sativex
- Before traveling abroad, you must check if it is legal to take this medicine into the countries you are visiting or passing through.
- Sativex is a controlled drug, so its legal status will vary depending on the country.
- Driving during treatment with Sativex may be illegal in some countries.
Sativex contains ethanol and propylene glycol
- This medicine contains up to 40 mg of ethanol (alcohol) per spray. The amount of alcohol in the maximum daily dose for most people (12 sprays) is equivalent to about two teaspoons (10 ml) of beer and approximately one teaspoon (5 ml) of wine. The small amount of alcohol in this medicine will not have a noticeable effect. The alcohol content should be taken into account in the case of pregnant or breastfeeding women, children, and high-risk groups, such as patients with liver disease or epilepsy.
- This medicine contains 52 mg of propylene glycol in each spray. Propylene glycol may cause skin irritation.
3. How to use Sativex
Follow the instructions for administration of this medicine contained in this leaflet or as directed by your doctor. Consult your doctor or pharmacist if you have any doubts.
Sativex is for oral use only: on the inside of the cheek or under the tongue.
- You can use Sativex with or without food. However, using Sativex with food may affect the amount of medicine absorbed by the body. As much as possible, try to use Sativex in the same way in relation to food, so you will always get the same effect.
Opening the spray and preparing it for use
- Take the spray out of the refrigerator (see the section “Storing Sativex”).
- Write the date you open the spray on the label provided at the end of the leaflet. Once opened, do not use the spray for more than 6 weeks (42 days).
- Gently shake the spray container before use.
- Remove the protective cap.
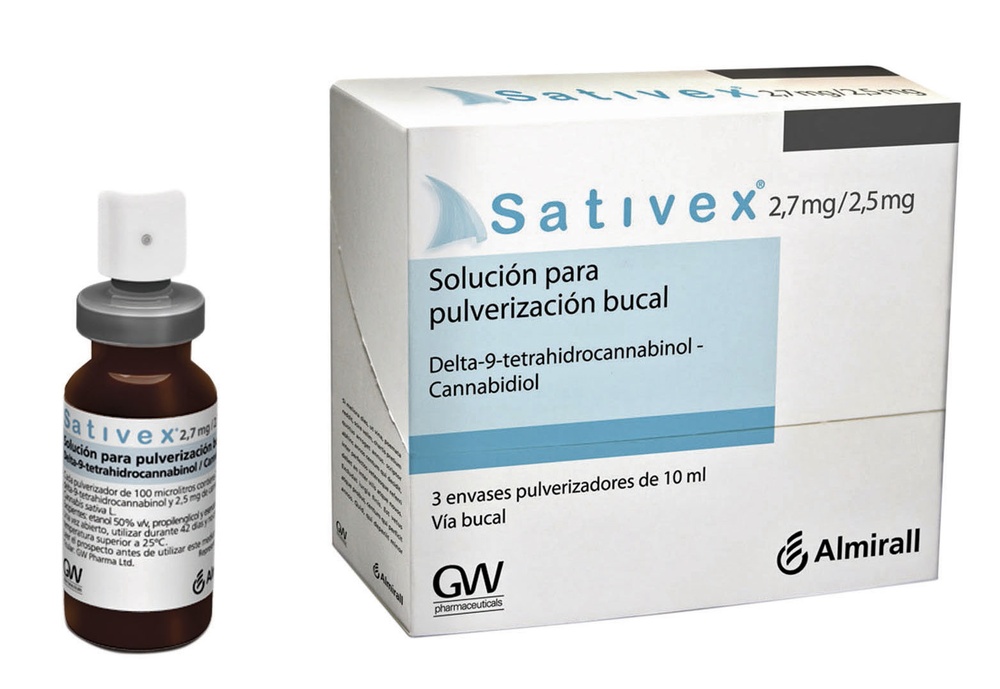
- Hold the spray between your thumb and index finger, and place your middle finger on the nozzle.
- Keep the container upright, then do 2 or 3 sprays onto a paper tissue until a fine spray appears. These sprays are to “prepare” the pump and make sure it is working correctly.
- The spray is now ready to use. You do not need to do any more preparation sprays until you open a new spray container.
Using the spray
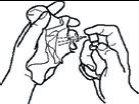
- Hold the spray between your thumb and index finger, and place your middle finger on the nozzle.
- Keep it upright and direct it towards your mouth. Place the nozzle under your tongue or towards the inside of your cheek. Vary the place where you spray to avoid discomfort in one area.
- Press the nozzle firmly. Do not do more than one spray at a time, even if it seems like a small amount is sprayed.
- Put the protective cap back on.
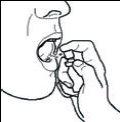
If you accidentally spray into your eyes, rinse them immediately with water.
- Do not inhale the product.
- Do not apply the product near children or animals.
- Do not use the spray near open flames or heat sources.
Determining the right dose
The number of sprays you need per day depends only on you. Each person needs a different number of sprays to get the most relief from muscle stiffness with the fewest side effects.
- When you start treatment with Sativex, you should follow the days and hours indicated in the table provided below to determine what is the optimal number of sprays for you.
- Stop increasing the sprays when you have determined the optimal number of sprays for you.This may take a few days to 2 weeks. Do the same number of sprays each day. Distribute the sprays evenly throughout the day.
- Do not do more than one spray at a time. You should always wait at least 15 minutes between each spray.
- Do not overexert yourself during the first few days of using Sativex until you know how it affects you.
- If you start to feel side effects (usually dizziness), do one spray less each day until you get the most relief from symptoms with the fewest side effects.
- Once you have determined what is the optimal number of sprays for you, do the same number of sprays each day. Distribute the sprays evenly throughout the day, according to your needs. However, you should always wait at least 15 minutes between each spray.
Number of sprays | |||
Day | Morning (between waking up and 12 noon) | Afternoon (between 4 pm and bedtime) | Total sprays per day |
Day 1 | 0 | 1 | 1 |
Day 2 | 0 | 1 | 1 |
Day 3 | 0 | 2 | 2 |
Day 4 | 0 | 2 | 2 |
Day 5 | 1 | 2 | 3 |
Day 6 | 1 | 3 | 4 |
Day 7 | 1 | 4 | 5 |
Day 8 | 2 | 4 | 6 |
Day 9 | 2 | 5 | 7 |
Day 10 | 3 | 5 | 8 |
Day 11 | 3 | 6 | 9 |
Day 12 | 4 | 6 | 10 |
Day 13 | 4 | 7 | 11 |
Day 14 | 5 | 7 | 12 |
Do not take more than 12 sprays per day, unless your doctor tells you to.
If you use more Sativex than you should
If you accidentally use more medicine than you should, you may:
- see or hear things that do not exist (hallucinations)
- feel dizzy, drowsy, or confused
- notice changes in heart rate
If you have used more Sativex than you should, tell your doctor or pharmacist. You can also contact the Toxicology Information Service, phone 91 562 04 20, stating the medicine and the amount used.
If you forget to use Sativex
- If you forget a dose, use a spray as soon as possible or when you notice you need a spray.
- Do not use 2 sprays at the same time to make up for a forgotten dose.
How to know if the spray is almost empty
Once you have done the 3 preparation sprays, the spray contains 90 measured sprays. When the spray starts to run out, the sound of the spray may change. The spray may also have a different taste. This is because the spray is almost empty. When this happens, you should open a new spray container.
If you stop using Sativex
If for any reason you decide to stop treatment with Sativex, tell your doctor or pharmacist. If you stop treatment suddenly, you may experience effects such as disturbed sleep, appetite, or mood for a short period. Generally, muscle stiffness will return gradually when you stop treatment with Sativex.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, Sativex can cause adverse effects, although not all people will experience them.
Stop taking this medication and consult your doctor or go directly to the hospital if
you experience any of the following serious adverse effects; you will be monitored
until the symptoms disappear:
- Seeing or hearing things that do not exist (hallucinations)
- Belief in false ideas
- Feeling that others are against you
- Suicidal thoughts
- Feeling depressed or confused
- Feeling overexcited or losing touch with reality
The following adverse effects are more frequent when starting treatment. In most cases, they are quite mild and usually disappear after a few days.
- If you experience any of the following adverse effects, use fewer sprays or stop using Sativex until you feel normal again.
- When you resume using the medication, use the number of sprays with which you did not experience adverse effects.
If you experience adverse effects, consult your doctor or pharmacist, even if they are adverse effects that do not appear in this prospectus.
Very Common (affects more than 1 in 10 people)
- Feeling of dizziness or fatigue
Common (affects less than 1 in 10 people)
- Memory or concentration problems
- Drowsiness or dizziness
- Blurred vision
- Difficulty speaking
- Increased or decreased appetite
- Alteration of taste or dry mouth
- Constipation or diarrhea
- Feeling of nausea or vomiting
- Mouth disorders, including burning, pain, or mouth ulcers
- Lack of energy, feeling of weakness, or general malaise
- Abnormal feeling or intoxication
- Loss of balance or falls
Uncommon (affects less than 1 in 100 people)
- Fainting
- Changes in pulse frequency, heart rate, or blood pressure
- Throat pain or irritation
- Abdominal pain
- Change in the color of the mouth or teeth
- Irritation at the Sativex spray site
- Redness or inflammation of the mouth or peeling of its interior. Do not continue spraying in these areas.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Sativex
- Keep this medication out of sight and reach of children.
- Do not use this medication after the expiration date shown on the packaging. The expiration date is the last day of the month indicated.
- Before use, store the Sativex container in a vertical position, inside its case, in the refrigerator (between 2°C and 8°C). If not stored in the refrigerator, it will become unstable and lose its desired effect.
- Once opened, store Sativex in a vertical position at a temperature below 25°C.
- Do not use Sativex once opened for more than 42 days.
- Medicines should not be thrown away through drains or into the trash. Deposit the containers and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Sativex Composition
- The active ingredients are cannabis extracts. 1 ml contains 38-44 mg and 35-42 mg of two extracts of Cannabis sativa L., leaf and flower, which correspond to 27 mg/ml of delta-9-tetrahydrocannabinol (THC) and 25 mg/ml of cannabidiol (CBD). Each 100 microliter spray contains 2.7 mg of THC and 2.5 mg of CBD.
- The other components are ethanol, propylene glycol, and peppermint essence.
Product Appearance and Package Contents
Sativex is a yellowish-brown liquid inside a 10 ml glass container equipped with a spray pump. The pump is protected with a plastic cap.
The number of measured sprays in the container is 90 (after the 3 preparation sprays).
Sativex is available in boxes containing 3 spray containers.
Marketing Authorization Holder
Jazz Pharmaceuticals Ireland Ltd
5th Floor
Waterloo Exchange
Waterloo Road
Dublin
D04 E5W7
Ireland
Manufacturer
Jazz Pharmaceuticals Netherlands B.V.
Stationsplein 13A, 3818 LE, Amersfoort, Netherlands
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Almirall, S.A.
General Mitre, 151 08022 Barcelona, Spain
Phone: 932913000
Email: [email protected]
Date of the last revision of this prospectus: 11/2024
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SATIVEX 2.7 mg/2.5 mg ORAL BUCCAL SPRAY SOLUTIONDosage form: TABLET, 125 mgActive substance: Other analgesics and antipyreticsManufacturer: Laboratorio Stada S.L.Prescription not requiredDosage form: INJECTABLE PERFUSION, 100 µgActive substance: ziconotideManufacturer: Esteve Pharmaceuticals GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 100 µgActive substance: ziconotideManufacturer: Esteve Pharmaceuticals GmbhPrescription required
Online doctors for SATIVEX 2.7 mg/2.5 mg ORAL BUCCAL SPRAY SOLUTION
Discuss questions about SATIVEX 2.7 mg/2.5 mg ORAL BUCCAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















