
RUCONEST 2100 U POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar RUCONEST 2100 U POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ruconest 2100 unidades de polvo y disolvente para solución inyectable
conestat alfa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4
Contenido del prospecto
- Qué es Ruconest y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ruconest
- Cómo usar Ruconest
- Posibles efectos adversos
- Conservación de Ruconest
- Contenido del envase e información adicional
1. Qué es Ruconest y para qué se utiliza
Ruconest contiene conestat alfa como principio activo. El conestat alfa es una forma recombinante (no hemoderivada) del inhibidor de C1 humano (rhC1-INH).
Ruconest debe ser usado por adultos, adolescentes y niños (a partir de 2 años) con un trastorno sanguíneo hereditario raro que se denomina angioedema hereditario (AEH). Estos pacientes presentan escasez de la proteína inhibidora de C1 en su sangre, lo cual puede provocar episodios repetidos de hinchazón, dolor en el abdomen, dificultad para respirar y otros síntomas.
La administración de Ruconest resuelve la escasez de inhibidor de C1 y permite una reducción de los síntomas de las crisis agudas de AEH.
2. Qué necesita saber antes de empezar a usar Ruconest
No use Ruconest:
- Si es o se considera alérgico a los conejos
- Si es alérgico a conestat alfa o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Ruconest.
Si experimenta reacciones alérgicas, como habones, exantema, picor, mareo, sibilancias, dificultad para respirar o hinchazón de la lengua tras la administración de Ruconest, solicite asistencia médica de emergencia para que se traten urgentemente los síntomas de la reacción alérgica.
Niños y adolescentes
No administre este medicamento a niños menores de 2 años. Ruconest no ha sido estudiado en niños menores de 5 años. Su médico determinará si el tratamiento con Ruconest es apropiado para su hijo. Se requiere supervisión adicional de su hijo para detectar síntomas de reacciones alérgicas durante y después de la administración.
Uso de Ruconest con otros medicamentos
Informe a su médico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Si recibe activador tisular del plasminógeno como tratamiento agudo para la prevención de coágulos sanguíneos (tratamiento anticoagulante), no debe usar Ruconest al mismo tiempo.
Embarazo y lactancia
No se recomienda administrar Ruconest durante el embarazo o la lactancia.
Si tiene intención de quedarse embarazada, consulte a su médico antes de utilizar Ruconest.
Conducción y uso de máquinas
No conduzca ni maneje máquinas si se siente mareado o le duele la cabeza después de utilizar Ruconest.
Ruconest contiene sodio (19,5 mg por vial)
Los pacientes con dietas pobres en sodio deben tener en cuenta que este medicamento contiene 19,5 mg de sodio por vial.
3. Cómo usar Ruconest
El tratamiento con Ruconest lo iniciará un médico especializado en el diagnóstico y tratamiento de angioedema hereditario.
Ruconest debe ser administrado por un profesional sanitario hasta que usted o su cuidador hayan recibido la formación necesaria y sean capaces de administrar Ruconest.
Use siempre este medicamento exactamente como se describe en este prospecto o según las indicaciones de su médico o enfermero. Consulte siempre a su médico o enfermero en caso de duda.
Ruconest se administra en una vena durante aproximadamente 5 minutos. Su dosis tendrá efecto en función de su peso corporal.
La mayor parte de las veces basta con una dosis. Podría administrarse una dosis adicional si sus síntomas no mejoran después de 120 minutos (para adultos y adolescentes) o 60 minutos (para niños). No se podrán administrar más de dos dosis, calculadas según el paso 7, en un plazo de 24 horas.
Usted o su cuidador pueden inyectar Ruconest solo después de recibir las instrucciones adecuadas y la formación necesaria por parte de su médico o enfermero.
Instrucciones de uso
No mezcle ni administre Ruconest con otros medicamentos o soluciones. A continuación se describe cómo se debe preparar y administrar la solución Ruconest.
Antes de empezar
- Asegúrese de que el envase está íntegro y contiene todos los componentes especificados en la sección 6 de este prospecto.
- Además del envase, se requiere lo siguiente:
- Un torniquete
- Esparadrapo para sujetar la aguja
- Inspeccione los viales y otros componentes.
- Todos los viales deben estar precintados con una tapa de plástico y un tapón de aluminio sin daños visibles, como grietas en el vidrio.
- Compruebe la fecha de caducidad. No use ningún componente del kit después de la fecha de caducidad indicada en el embalaje exterior grande.
En una sola caja, los distintos componentes pueden tener fechas de caducidad diferentes. La fecha de caducidad del embalaje exterior refleja la fecha del componente con el período de validez menor.
- Espere hasta que el número de viales de polvo y disolvente requeridos según el paso 1 alcancen la temperatura ambiente.
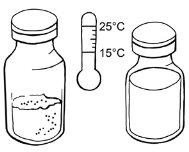
Preparación de la solución
Paso 1:Limpieza y otros requisitos
- Lávese bien las manos.
- Coloque los viales de polvo y disolvente requeridos en una superficie plana y limpia.
- Peso corporal de 42 kg o menos: 1 vial de polvo y 1 vial de disolvente
- Peso corporal superior a 42 kg: 2 viales de polvo y 2 viales de disolvente
- Coloque los adaptadores de viales en la superficie de trabajo. No retire el envase de los adaptadores.
- 2 adaptadores para un vial de polvo y 1 vial de disolvente
- 4 adaptadores para 2 viales de polvo y 2 viales de disolvente
- Coloque las jeringas en la superficie de trabajo. No retire el envase de las jeringas.
- 1 jeringa para 1 vial de polvo y 1 vial de disolvente
- 2 jeringas para 2 viales de polvo y 2 viales de disolvente
Paso 2:Desinfección de los tapones de los viales
- Retire el tapón a presión de plástico de los viales de polvo y disolvente.
- Use una toallita con alcohol para desinfectar todos los tapones de los viales y espere como mínimo 30 segundos hasta que los tapones se hayan secado.
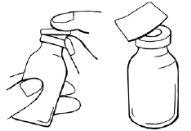
- Después de la desinfección, no toque los tapones con las manos ni ningún objeto.
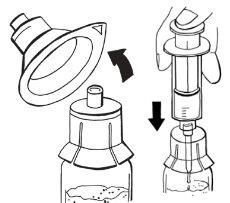
Paso 3:Montaje de los adaptadores en los viales
- Sujete un adaptador envasado con una mano y retire la tapa. El adaptador debe permanecer en su envase de plástico.
- Coloque el adaptador en un vial de polvo y perfore el tapón hasta que se acople al cuello del vial.
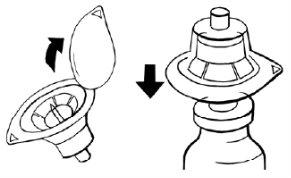
- Deje el envase del adaptador hasta que conecte la jeringa según los pasos 4 y 5.
- Repita los pasos anteriores para montar un adaptador en el vial de disolvente. Todos los adaptadores suministrados en el envase son idénticos.
- Si necesita usar un segundo vial de polvo y disolvente, repita los pasos anteriores.
Paso 4:Extracción de disolvente
- Extraiga una jeringa estéril de su envase.
- Retire el envase del adaptador del vial de disolvente.
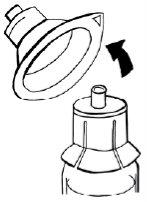
- Sujete el adaptador con una mano. Con la otra mano, acople la jeringa y gírela hacia la izquierda hasta que se detenga para asegurarla.
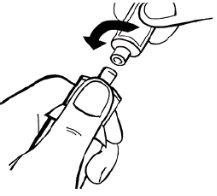
- Invierta por completo el vial de disolvente junto con el adaptador y la jeringa. Mientras lo mantiene en posición vertical, inyecte lentamente 14 ml de disolvente.
Si se forman burbujas, debe minimizarlas en la medida de lo posible. Para ello, dé un ligero toque en la jeringa y ejerza una ligera presión empujando el émbolo en la jeringa. Siga llenando la jeringa hasta alcanzar los 14 ml de disolvente.
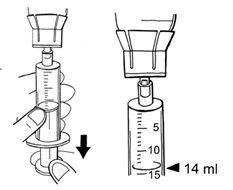
- Para desconectar la jeringa del adaptador, gire hacia la izquierda.
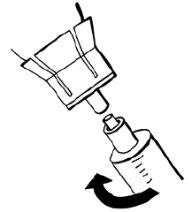
- Deje el resto del disolvente en el vial y deseche el vial.
- Coloque la jeringa en la superficie de trabajo y procure no tocar la superficie ni ningún otro objeto con la punta de la jeringa.
Paso 5:Adición de disolvente al polvo y disolución
- Retire el envase del adaptador del vial de polvo.
- Tome la jeringa con disolvente que ha preparado en el paso 4.
- Sujete el adaptador con la otra mano y acople la jeringa. Para sujetar bien la jeringa, gírela hacia la derecha hasta que se detenga.
- Presione el disolvente lentamente con un solo movimiento para introducirlo en el vial de polvo y minimizar así la formación de espuma.
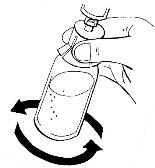
- Deje la jeringa en el adaptador y gire con cuidado el vial durante aproximadamente medio minuto. No lo agite.
Después de darle vueltas, deje el vial en la superficie durante varios minutos hasta que la solución sea transparente. Si hay polvo no disuelto, repita el procedimiento.
- Repita los pasos 4 y 5 si necesita preparar una segunda solución.
Paso 6:Comprobación de soluciones preparadas
- Compruebe si el polvo de los viales se ha disuelto por completoy si el émbolo está en el fondo de la jeringa.
- Una vez disuelto el polvo, la solución debe ser transparente e incolora.
- No use la solución preparada si está turbia, contiene partículas o ha cambiado de color. Informe al profesional sanitario en este caso. Es aceptable la formación de espuma en pequeñas cantidades.
 Paso 7:Extracción de la solución preparada
Paso 7:Extracción de la solución preparada
- Calcule los milímetros de solución preparada que se van a inyectar.
Peso corporal | Mililitros de solución preparada que se van a inyectar |
Inferior a 84 kg | Peso corporal en kg dividido por tres |
84 kg y superior | 28 ml |
- Inyecte el volumen de solución preparada mientras mantiene la jeringa en posición vertical. Si ha preparado:
- un vial con solución, extraiga el volumen calculado
- dos viales y su peso corporal es inferior a 84 kg, extraiga una cantidad similar:
- 14 ml del primer vial
- del segundo vial, la diferencia entre el volumen calculado y los 14 ml del primer vial
- dos viales y su peso corporal es de 84 kg o más, extraiga 14 ml de cada vial en cada jeringa
Si se forman burbujas, debe minimizarlas en la medida de lo posible. Para ello, dé un ligero toque en la jeringa y ejerza una ligera presión empujando el émbolo en la jeringa. Siga llenando la jeringa hasta alcanzar el volumen requerido.
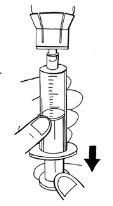
- No supere en ningún caso el volumen de 14 ml por jeringa.
- Para soltar la jeringa, gírela hacia la izquierda y deseche los viales junto con el adaptador.
- Coloque la jeringa en la superficie de trabajo y procure no tocar la superficie ni ningún otro objeto con la punta de la jeringa.
Paso 8:Comprobación de jeringas preparadas
- Vuelva a comprobar si el volumen de las jeringas preparadas en el paso7 es correcto.
Administración en una vena
Es muy importante que la solución preparada se inyecte directamente en una vena y no en una arteria o en el tejido circundante.
Inyecte la solución Ruconest de inmediato tras la preparación, preferiblemente sentado.
Paso 9:Componentes requeridos
- Compruebe si todos los componentes necesarios se encuentran en la superficie de trabajo:
-1 o 2 jeringas con la solución preparada
-1 equipo de perfusión con una aguja de 25 G
-1 toallita con alcohol
-1 gasa no tejido estéril
-1 esparadrapo autoadhesivo
-1 torniquete
-1 esparadrapo para sujetar la aguja
Paso 10:Preparación del equipo de perfusión
- Retire el tapón de rosca del extremo del equipo de perfusión. Este es el extremo sin aguja.
- Sujete este extremo con una mano, acople la punta de la jeringa y asegúrela girando hacia la derecha hasta que se detenga.
- Sostenga la jeringa con la punta hacia arriba. Presione ligeramente el émbolo de la jeringa para llenar con cuidado el equipo de perfusión con la solución preparada.
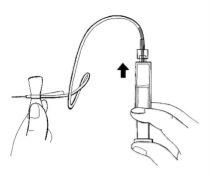
- Compruebe que no haya aire en la jeringa, el tubo de perfusión o la aguja.
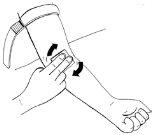
Paso 11:Preparación del punto de inyección
- Coloque el tornique sobre el punto de inyección, preferiblemente en la parte central de la parte superior del brazo. Apriete para comprimir la vena. Puede lograr este efecto apretando el puño.
- Palpe con la otra mano la vena adecuada.
- Desinfecte bien el punto de inyección con una toallita con alcohol y deje que la piel
se seque.
Paso 12:Administración de la solución preparada
- Retire la funda de la aguja.
- Inserte con cuidado la aguja del equipo de perfusión, en el ángulo más plano posible, en la vena.
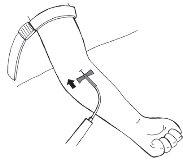
- Sujete la aguja con el esparadrapo, aproximadamente 7 cm de largo, sobre las aletas de la aguja.
- Tire hacia atrás con cuidado y ligeramente del émbolo de la jeringa hasta ver entrar la sangre en el tubo para asegurarse de que la aguja está en la vena.
- Suelte el torniquete.
- Si no hay sangre en el tubo, retire la aguja, repita todos los pasos desde el paso 11 y vuelva a colocar la aguja.
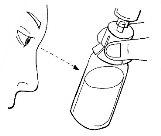
- Si hay sangre, inyecte con cuidado la solución en la vena, como se muestra en la imagen. Inyecte durante 5 minutos aproximadamente.
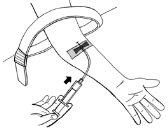
- Si ha preparado dos jeringas:
-Doble el tubo cerca del conector del equipo de perfusión para evitar la inversión del flujo
-Desenrosque la jeringa vacía del equipo de perfusión y cámbiela de inmediato por la segunda jeringa.
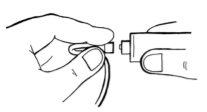
-Despliegue el tubo e inyecte con cuidado esta solución de forma similar a la primera jeringa.
Paso 13: Después de la administración
- Retire con cuidado el esparadrapo que sujeta la aguja y retire la aguja de la vena.
- Justo después de retirar la aguja, presione la gasa estéril sobre el punto de inyección durante unos minutos para reducir el sangrado.
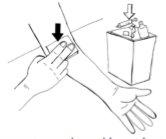
- A continuación, coloque el esparadrapo autoadhesivo en el punto de inyección.
- Pliegue la funda de protección amarilla sobre la aguja.
- Deseche de forma segura el equipo de perfusión usado con la aguja, la solución no usada, la jeringa y el vial vacío en un contenedor de residuos apropiado, ya que estos materiales pueden provocar lesiones si no se desechan correctamente. No reutilice el equipo.
Paso 14: Documentación de la administración
Registre lo siguiente (por ejemplo, en su diario):
- Fecha y hora de administración
- Número de lote impreso en la etiqueta del vial de polvo
Si usa más Ruconest del que debe
Póngase en contacto con su médico o con el hospital más próximo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si sus síntomas empeoran y/o presenta exantema, hormigueo, dificultad para respirar o hinchazón de la cara o la lengua, acuda al médico inmediatamente. Estos síntomas pueden indicar que ha desarrollado alergia a Ruconest.
Durante el tratamiento con Ruconest pueden aparecer algunos efectos secundarios:
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Náuseas
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- Dolor abdominal, diarrea
- Sensación de hormigueo, pinchazos o entumecimiento de la boca
- Dolor de cabeza, mareo
- Disminución del sentido del tacto o la sensibilidad en la piel o las extremidades
- Irritación de garganta
- Urticaria (habones)
- Hinchazón de las orejas o de la zona alrededor de las orejas
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ruconest
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en la etiqueta del vial después de EXP. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC.
Conservar el vial de polvo en la caja del vial para protegerlo de la luz.
Antes de administrar Ruconest, se debe disolver en el disolvente incluido en el envase (ver sección 3). Una vez reconstituido, el producto debe utilizarse de inmediato.
No utilice este medicamento si, tras la disolución, observa que la solución contiene partículas o si la solución está descolorida. La formación de espuma es aceptable en pequeñas cantidades.
6. Contenido del envase e información adicional
Composición de Ruconest
Vial de polvo:
- El principio activo es conestat alfa. Un vial de polvo contiene 2100 unidades (U) de conestat alfa. Esto equivale a 2100 unidades por 14 ml después de la reconstitución, o a una concentración de 150 unidades/ml.
- Los demás componentes son sacarosa, citrato de sodio (E331) y ácido cítrico.
Vial de disolvente:
- El ingrediente del disolvente es agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Ruconest se presenta en un único vial de vidrio que contiene un polvo blanco a blanquecino para solución inyectable además de un vial de vidrio con un disolvente incoloro transparente para disolver el polvo. Después de la disolución del polvo en agua para preparaciones inyectables, la solución es transparente e incolora.
Ruconest se suministra como un kit en una caja que contiene:
- 1 vial de 2100 U de polvo
- 1 vial de 20 ml de disolvente
- 2 adaptadores de viales
- 1 jeringa
- 1 equipo de perfusión con tubo de 35 cm y aguja de 25 G
- 2 toallitas con alcohol
- 1 gasa no tejido estéril
- 1 esparadrapo autoadhesivo
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Pharming Group N.V.
Darwinweg 24
2333 CR Leiden
Países Bajos
Responsable de la fabricación:
Pharming Technologies B.V.
Darwinweg 24
2333 CR Leiden
Países Bajos
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu/.
------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
POSOLOGÍA Y FORMA DE ADMINISTRACIÓN
Posología
Peso corporal hasta 84 kg
- Una inyección intravenosa de 50 U/kg de peso corporal.
Peso corporal de 84 kg o mayor
- Una inyección intravenosa de 4.200 U (dos viales).
En la mayor parte de los casos, una sola inyección de Ruconest es suficiente para el tratamiento de una crisis aguda de angioedema.
Si la respuesta clínica es insuficiente, podrá administrarse una segunda dosis (50 U/kg de peso corporal hasta 4200 U).
No se podrán administrar más de dos dosis en un plazo de 24 horas.
Cálculo de la dosis
Determinar el peso corporal del paciente.
Peso corporal hasta 84 kg
- En los pacientes de hasta 84 kg de peso, el volumen de administración necesario se calculará con la siguiente fórmula:
Volumen a administrar (ml) | = | Peso corporal (kg) x 50 (U/kg) 150 (U/ml) | = | Peso corporal (kg) 3 |
Peso corporal de 84 kg o mayor
- En los pacientes de 84 kg o superior, el volumen de administración necesario es de 28 ml, equivalente a 4.200 U (2 viales).
Reconstituya cada vialcon 14 ml de agua para preparaciones inyectables (ver sección Reconstitución más adelante).
La solución reconstituida en cada vial contiene 2.100 U de conestat alfa a 150 U/ml.
El volumen necesario de la solución reconstituida se debe administrar mediante inyección intravenosa lenta durante aproximadamente 5 minutos.
PRECAUCIONES ESPECIALES DE ELIMINACIÓN Y OTRAS MANIPULACIONES
Preparación y manipulación
Cada vial de Ruconest es para un solo uso.
Ruconest se debe administrar por vía intravenosa después de la reconstitución con agua para preparaciones inyectables. Se utilizará una técnica aséptica para la reconstitución, la combinación y la mezcla de soluciones.
Reconstitución
- Cada vial de Ruconest (2100 U) se debe reconstituir con 14 ml de agua para preparaciones inyectables.
- Desinfecte los tapones de caucho de los viales de polvo y disolvente, y coloque un adaptador de viales en cada vial de disolvente y polvo para acoplarlo al cuello del vial.
- Conecte la jeringa al adaptador del vial de disolvente y gírela hacia la derecha hasta acoplarla. Para soltar la jeringa del adaptador, gírela hacia la izquierda y deseche el vial junto con el adaptador.
- Conecte la jeringa con disolvente al adaptador del vial de polvo y gírela hacia la derecha hasta acoplarla. El disolvente se debe añadir lentamente para evitar un impacto fuerte en el polvo y mezclar suavemente para minimizar la formación de espuma en la solución. Deje la jeringa en el adaptador. Repita los pasos 3 y 4 si necesita preparar una segunda solución (esto requiere un segundo envase).
- La solución reconstituida contiene 150 U/ml de conestat alfa y es una solución incolora transparente. La solución reconstituida en cada vial se debe examinar en busca de partículas y cambios de color. No se debe utilizar una solución que presente partículas o cambios de color. Es aceptable la formación de pequeñas cantidades de espuma. Este medicamento se debe utilizar inmediatamente.
Administración
- Inyecte el volumen requerido de la solución preparada. No supere en ningún caso 14 ml por jeringa. Para soltar la jeringa, gire hacia la derecha y deseche el vial junto con el adaptador.
- Acople el equipo de perfusión a la jeringa y gire hacia la derecha para bloquearlo. Sujete la jeringa con la punta hacia arriba y presione ligeramente el émbolo para llenar de solución el equipo de perfusión.
- Desinfecte el punto de inyección con una toallita con alcohol. Retire la funda de la aguja del equipo de perfusión e introduzca con cuidado la aguja en la vena.
- Asegúrese de soltar el torniquete. Inyecte con cuidado la solución en la vena (durante 5 minutos aproximadamente).
- En caso de preparar dos jeringas, doble el tubo para evitar la inversión del flujo, desenrosque la jeringa vacía del equipo de perfusión (hacia la izquierda) y sustitúyala de inmediato por la segunda jeringa. Inyecte con cuidado la solución de la segunda jeringa.
Eliminación
Elimine de forma segura el equipo de perfusión usado con la aguja, la solución no usada, la jeringa y el vial vacío en un contenedor de residuos médicos apropiado, ya que estos materiales pueden provocar lesiones si no se desechan correctamente. No reutilice el equipo.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RUCONEST 2100 U POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 2100 UPrincipio activo: Conestat alfaFabricante: Pharming Group N.V.Requiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: Drugs used in hereditary angioedemaFabricante: Csl Behring GmbhRequiere recetaForma farmacéutica: INYECTABLE, 1500 UIPrincipio activo: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequiere receta
Médicos online para RUCONEST 2100 U POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RUCONEST 2100 U POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















