
RIVASTIGMINA ABABOR 2 MG/ML SOLUCION ORAL EFG

Cómo usar RIVASTIGMINA ABABOR 2 MG/ML SOLUCION ORAL EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Rivastigmina Ababor 2 mg/ml solución oral EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no parecen en este prospecto.Ver sección 4.
Contenido del prospecto
- Qué es Rivastigmina Ababor y para qué se utiliza
2 Qué necesita saber antes de empezar a tomar Rivastigmina Ababor
- Cómo tomar Rivastigmina Ababor
- Posibles efectos adversos
- Conservación de Rivastigmina Ababor
- Contenido e información adicional
1. Qué es Rivastigmina Ababor y para qué se utiliza
El principio activo es Rivastigmina.
Rivastigmina pertenece al grupo de sustancias denominadas inhibidores de la colinesterasa.
En pacientes con demencia por Alzheimer o demencia debida a enfermedad de Parkinson, ciertas células nerviosas mueren en el cerebro, resultando en niveles bajos del neurotransmisor acetilcolina (una sustancia que permite que las células nerviosas se comuniquen entre sí). Rivastigmina actúa bloqueando las enzimas que descomponen la acetilcolina: la acetilcolinesterasa y la butirilcolinesterasa. Al bloquear estas enzimas, rivastigmina permite que los niveles de acetilcolina aumenten en el cerebro, ayudando a reducir los síntomas de la enfermedad de Alzheimer y demencia asociada a enfermedad de Parkinson.
Rivastigmina Ababor se utiliza para el tratamiento en pacientes adultos con demencia por Alzheimer de leve a moderadamente grave , un trastorno cerebral progresivo que gradualmente afecta la memoria, capacidad intelectual y la conducta.También se puede utilizar para el tratamiento de la demencia en pacientes adultos con enfermedad de Parkinson.
2. Qué necesita saber antes de tomar Rivastigmina Ababor
No tome Rivastigmina Ababor
- si es alérgico a rivastigmina o a cualquiera de los demás componentes de
este medicamento (incluidos en la sección 6).
Si le ocurre esto, dígaselo a su médico y deje de tomar Rivastigmina Ababor
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar Rivastigmina Ababor.
- si tiene o ha tenido alguna vez ritmo cardíaco irregular o lento
- si tiene o ha tenido alguna vez úlcera de estómago activa
- si tiene o ha tenido alguna vez dificultades al orinar
- si tiene o ha tenido alguna vez crisis epilépticas (ataques o convulsiones)
- si tiene o ha tenido alguna vez asma o una enfermedad respiratoria grave
- si tiene o ha tenido alguna vez alteración de las funciones del riñón
- si tiene o ha tenido alguna vez alteración de las funciones del hígado
- si sufre temblores
- si tiene peso corporal bajo
- si experimenta reacciones gastrointestinales tales como náuseas, vómitos y diarrea. Puede deshidratarse (perder mucho líquido) si el vómito o diarrea son prolongados
Si se encuentra en alguna de estas situaciones, puede que su médico considere necesario realizar un mayor seguimiento mientras esté en tratamiento.
Si olvidó tomar Rivastigmina Ababor durante más de tres días, no tome la siguiente dosis hasta que no consulte con
su médico.
Niños y adolescentes
No hay ningún uso pertinente de Rivastigmina Ababor en la población pediátrica para el tratamiento de la enfermedad del Alzheimer.
Uso de Rivastigmina Ababor con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Rivastigmina no deberá administrarse al mismo tiempo que otros medicamentos con efectos similares a los suyos. Rivastigmina puede interferir con medicamentos anticolinérgicos (medicamentos utilizados para aliviar retortijones o espasmos estomacales, para tratar la enfermedad de Parkinson o para prevenir
el mareo de un viaje).
Rivastigmina Ababor no debería administrarse al mismo tiempo que metoclopramida (un medicamento utilizado para aliviar o prevenir las náuseas y vómitos). Tomar los dos medicamentos juntos podría causar problemas tales como extremidades rígidas y manos temblorosas.
En caso de que tenga que someterse a una intervención quirúrgica mientras está tomando rivastigmina, informe a su médico antes de que se le administre algún anestésico, ya que rivastigmina puede exagerar los efectos de algunos relajantes musculares durante la anestesia.
Se debe tener precaución cuando se toma rivastigmina junto betabloqueantes (medicamentos como el atenolol que se usa para la hipertensión, la angina y otras condiciones de corazón). Tomar los dos medicamentos juntos podría causar problemas tales como disminución de los latidos del corazón (bradicardia) provocando desmayos o pérdida de la conciencia.
Embarazo, lactancia y fertilidad
Si está embarazada o en período de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Si está embarazada, es necesario evaluar los beneficios del uso de Rivastigmina Ababor, frente a los posibles efectos adversos para el feto.
Es preferible evitar el uso de Rivastigmina Ababor durante el embarazo, a menos que sea claramente necesario. Informe a su médico si se queda embarazada durante el tratamiento.
Las mujeres en tratamiento con Rivastigmina Ababor no deberán amamantar a sus hijos.
Conducción y uso de máquinas
Su enfermedad puede afectar la capacidad de conducir o utilizar maquinaria y no debe llevar a cabo estas actividades a menos que su médico le indique que es seguro realizarlas.
Rivastigmina Ababor puede causar mareos y somnolencia, principalmente al inicio del tratamiento o al aumentar la dosis. Si experimenta estos efectos, no deberá conducir, utilizar maquinaria o desarrollar tareas que requieran su atención.
Información importante sobre alguno de los componentes de Rivastigmina Ababor 2 mg/ml solución oral
Uno de los excipientes en Rivastigmina Ababor solución oral es el benzoato de sodio. Este medicamento puede ser irritante para la piel, ojos y membranas mucosas porque contiene ácido benzoico.
Rivastigmina Ababor también contiene 1,867 mg de sodio por ml de solución, lo que deberá tenerse en cuenta en el tratamiento de pacientes con dietas pobres en sodio.
3. Cómo tomar Rivastigmina Ababor
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Cómo comenzar el tratamiento
Su médico le indicará que dosis debe tomar de Rivastigmina
? El tratamiento normalmente comienza con una dosis baja
?Su médico le incrementará gradualmente la dosis dependiendo de cómo responda al tratamiento
? La dosis máxima que debe tomarse es de 6 mg dos veces al día
Su médico debe evaluar periódicamente si ejerce los efectos deseados. Su médico le controlará también el peso mientras esté tomando este medicamento
Si no ha tomado Rivastigmina durante más de tres días, no tome la siguiente dosis hasta que haya hablado con su médico.
Toma de este medicamento
? Informe a su cuidador de que está tomando Rivastigmina Ababor.
? Para que su medicamento ejerza el efecto deseado, debe tomarlo todos los días
? Rivastigmina Ababor debe tomarse dos veces al día con las comidas (por la mañana y por la
noche)
Cómo tomar este medicamento

- Preparar el bote y la jeringa:
? Extraer la jeringa de su funda protectora
? Presionar y girar el cierre de seguridad para niños para abrir el frasco.
- Meter el adaptador de la jeringa en el orificio del tapón
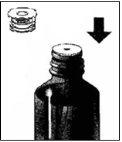
3 . Meter la jeringa en el adaptador
 ?
?
? Meter la cánula de la jeringa en el orificio del obturador.
- Llenar la jeringa
? Tirar del émbolo de la jeringa hasta que el líquido alcance la marca de la dosis que le ha prescrito su médico
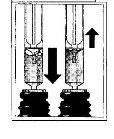
- Eliminar burbujas
? Eliminar las burbujas de gran tamaño con movimientos alternativos del émbolo.
? Un par de burbujas pequeñas no son importantes y no afectan a la dosis en ningún sentido.
? Comprobar que la dosis es la correcta
? Posteriormente retirar la jeringa del bote.
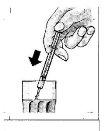
- Tomar su medicina
? Tomar la dosis directamente de la jeringa.
? Puede mezclarla también con agua en un vaso pequeño. Remover y beber la mezcla completamente.
- Tras el uso de la jeringa
? Limpiar la parte exterior de la jeringa y el adaptador con un pañuelo limpio.
? Posteriormente, devuelva la jeringa a su funda protectora.
? Ponga el cierre de seguridad para niños en el bote para cerrarlo.
Si toma más Rivastigmina Ababor del que debiera
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico, farmacéutico o llame al Servicio de Información toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad tomada.
Algunas personas que han tomado accidentalmente dosis superiores han sufrido náuseas, vómitos, diarrea, tensión arterial alta y alucinaciones. Puede producirse también un enlentecimiento de la frecuencia cardíaca y desmayos.
Si olvidó tomar Rivastigmina Ababor
Si olvida su dosis de rivastigmina Ababor, espere y tome la siguiente dosis a la hora habitual. No tome una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra pregunta sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento, puede producir efectos adversos, aunque no todas las personas los sufran.
La tendencia a notar efectos adversos es más frecuente al empezar a tomar su medicamento o al aumentar la dosis. Los efectos adversos desaparecerán de forma gradual muy probablemente a medida que su organismo vaya acostumbrándose al medicamento.
Muy frecuentes:(pueden afectar a más de 1 de cada 10 personas)
- Mareo
- Pérdida de apetito
- Problemas estomacales como náuseas, vómitos, diarrea
Frecuentes:(pueden afectar hasta 1 de cada 10 personas)
- Ansiedad
- Sudoración
- Dolor de cabeza
- Ardor
- Pérdida de peso
- Dolor de estómago
- Agitación
- Sensación de cansancio o debilidad
- Malestar general
- Temblor o sensación de confusión
- Disminución del apetito
- Pesadillas
Poco frecuentes:(pueden afectar hasta 1 de cada 100 personas)
- Depresión
- Dificultad para dormir
- Desmayos o caídas accidentales
- Cambios en la función hepática
Raros:(pueden afectar hasta 1 de cada 1000 personas)
- Dolor torácico
- Erupción cutánea
- Crisis epilépticas (ataques o convulsiones)
- Úlceras gástricas e intestinales
Muy raros :(pueden afectar hasta 1 de cada 10000 personas)
- Tensión arterial alta
- Infección del tracto urinario
- Alucinaciones
- Problemas del ritmo cardíaco (velocidad rápida o lenta)
- Hemorragia gastrointestinal (sangre en las heces o al vomitar)
- Inflamación del páncreas (dolor fuerte en la parte alta del estómago, frecuentemente acompañado de
náuseas y vómitos)
- Empeoramiento de la enfermedad de Parkinson o desarrollo de síntomas similares (rigidez muscular,
dificultad para realizar movimientos).
Frecuencia no conocida:(no puede estimarse a partir de los datos disponibles)
- Vómitos graves que pueden provocar una ruptura esofágica (parte del tubo digestivo que conecta la
boca con el estómago)
- Deshidratación (pérdida excesiva de líquido)
- Problemas hepáticos (piel amarillenta, amarillamiento del blanco de los ojos, oscurecimiento anormal
de la orina o náuseas, vómitos, cansancio y pérdida de apetito anormal)
- Agresión, sensación de inquietud
- Latido irregular de corazón
- Síndrome de Pisa (afección que conlleva una contracción muscular involuntaria y la inclinación anormal del cuerpo y la cabeza hacia un lado)
Pacientes con demencia asociada a la enfermedad de Parkinson
Estos pacientes experimentan algunos efectos adversos más frecuentemente así como efectos adversos adicionales:
Muy frecuentes:(pueden afectar a más de 1 de cada 10 personas)
- Temblor
- Desmayos
- Caídas accidentales
Frecuentes:(pueden afectar hasta 1 de cada 10 personas)
- Ansiedad
- Intranquilidad
- Ritmo cardiaco lento y rápido
- Dificultad para dormir
- Excesiva saliva y deshidratación
- Movimientos anormalmente lentos o incontrolables
- Empeoramiento de la enfermedad de Parkinson o desarrollo de síntomas similares (rigidez muscular,
dificultad para realizar movimientos y debilidad muscular)
Poco frecuentes:(pueden afectar hasta 1 de cada 100 personas)
- Latido cardíaco irregular y bajo control del movimiento
Frecuencia no conocida: (no puede estimarse a partir de los datos disponibles)
- Síndrome de Pisa (afección que conlleva una contracción muscular involuntaria y la inclinación anormal del cuerpo y la cabeza hacia un lado)
Se han observado las siguientes reacciones adversas adicionales con el uso de Rivastigmina parches transdérmicos, las cuales pueden ocurrir con la solución oral:
Frecuentes:
- Fiebre
- Confusión severa
- Incontinencia urinaria (incapacidad para retener la orina adecuadamente)
Poco frecuentes: (pueden afectar hasta 1 de cada 100 personas)
-Hiperactividad (elevado nivel de actividad, inquietud)
Frecuencia no conocida:(no puede estimarse a partir de los datos disponibles)
Reacción alérgica donde fue utilizado el parche, tales como ampollas o inflamación de la piel.
Si ocurren estos síntomas contacte con su médico ya que puede requerir asistencia médica.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://notificaram.es Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre seguridad de este medicamento.
5. Conservación de Rivastigmina Ababor
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No refrigerar.
Mantener el envase en posición vertical.
Usar Rivastigmina Ababor 2mg/ml solución oral en el mes posterior a la primera apertura del frasco.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
- El principio activo es hidrogenotartrato de rivastigmina equivalente a 2,0 mg de rivastigmina base.
- Los demás componentes son: benzoato sódico (E211), ácido cítrico monohidrato (E330), citrato sódico (E331), amarillo de quinoleína soluble en agua (E104), agua purificada.
Aspecto del producto y contenido del envase
Rivastigmina Ababor 2mg/ml solución oral se presenta en forma de solución transparente, amarilla (2,0 mg/ml rivastigmina base) de 50 o 120 ml en frascos de vidrio ámbar tipo III con cierre de seguridad para niños. Junto con la solución oral se incluye una jeringa para dosificación oral y adaptador para la jeringa en tubo de plástico.
Titular de la Autorización de Comercialización
Ababor Pharmaceuticals, S.L.
Chile, 4 - Edificio 1 - Oficina 1 - Las Matas
28290- Las Rozas. España
Responsable de la fabricación
Pharmanel Pharmaceuticals SA
60th km of Athens – Lamia Highway,
GR 320 09
Grecia
Y
Pharmathen SA
Dervenakion 6
Pallini 15351
Attiki, Grecia
Este prospecto ha sido autorizado en Septiembre 2015
Fecha de última revision de este prospecto: Enero 2025
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RIVASTIGMINA ABABOR 2 MG/ML SOLUCION ORAL EFGForma farmacéutica: PARCHE TRANSDERMICO, 13,3 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 4,6 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 9,5 mg/24 hPrincipio activo: RivastigminaFabricante: Esteve Pharmaceuticals S.A.Requiere receta
Médicos online para RIVASTIGMINA ABABOR 2 MG/ML SOLUCION ORAL EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RIVASTIGMINA ABABOR 2 MG/ML SOLUCION ORAL EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes













