РЕКАМБИС 900 МГ СУСПЕНЗІЯ ДЛЯ ІН'ЄКЦІЙ ПРОЛОНГОВАНОЇ ДІЇ

Запитайте лікаря про рецепт на РЕКАМБИС 900 МГ СУСПЕНЗІЯ ДЛЯ ІН'ЄКЦІЙ ПРОЛОНГОВАНОЇ ДІЇ

Інструкція із застосування РЕКАМБИС 900 МГ СУСПЕНЗІЯ ДЛЯ ІН'ЄКЦІЙ ПРОЛОНГОВАНОЇ ДІЇ
Введення
Опис: інформація для пацієнта
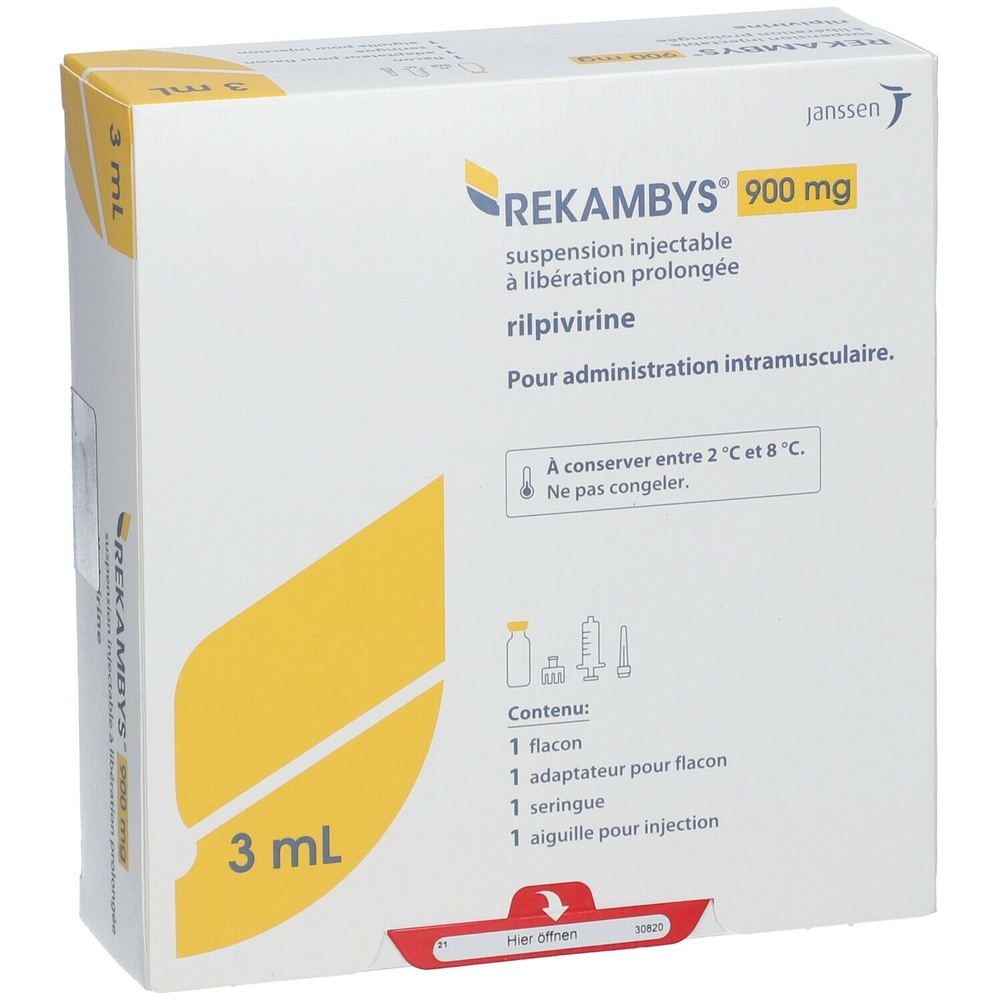
РЕКAMBYS 900 мг суспензія для ін'єкцій пролонгованої дії
рілпівіріна
Цей лікарський засіб підлягає додатковому моніторингу, що прискорить виявлення нової інформації про його безпеку. Ви можете допомогти, повідомивши про будь-які побічні ефекти, які ви можете мати. Остання частина розділу 4 містить інформацію про те, як повідомляти про ці побічні ефекти.
Прочитайте уважно весь опис перед тим, як почати використовувати цей лікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лікарський засіб призначений тільки вам, і не давайте його іншим людям, навіть якщо вони мають相同ні симптоми, оскільки це може їм нашкодити.
- Якщо ви 경험уєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке РЕКAMBYS і для чого він використовується
- Що потрібно знати перед тим, як почати використовувати РЕКAMBYS
- Як застосовується РЕКAMBYS
- Можливі побічні ефекти
- Збереження РЕКAMBYS
- Зміст упаковки та додаткова інформація
1. Що таке РЕКAMBYS і для чого він використовується
РЕКAMBYS містить активну речовину рілпівіріну. Він належить до групи лікарських засобів, які називаються інгібіторами зворотної транскриптази, неаналогами нуклеозидів (ІТІНАН), які використовуються для лікування вірусу імунодефіциту людини типу 1 (ВІЛ-1).
РЕКAMBYS діє разом з іншими лікарськими засобами проти ВІЛ, блокуючи здатність вірусу створювати копії самого себе. РЕКAMBYS для ін'єкцій не лікує інфекцію ВІЛ, але допомагає зменшити кількість ВІЛ у вашому організмі і тримати її на низькому рівні. Таким чином, він гальмує пошкодження імунної системи і розвиток інфекцій та захворювань, пов'язаних з СНІДом.
РЕКAMBYS завжди застосовується разом з іншим лікарським засобом проти ВІЛ, який називається каботегравіром для ін'єкцій. Вони застосовуються разом у дорослих від 18 років, чия інфекція ВІЛ-1 вже контролюється.
2. Що потрібно знати перед тим, як почати використовувати РЕКAMBYS
Не використовувати РЕКAMBYS, якщо ви алергічні на рілпівіріну або на будь-який інший компонент цього лікарського засобу (включно з розділом 6).
Не використовувати РЕКAMBYS, якщо ви приймаєте будь-який з наступних лікарських засобів, оскільки вони можуть вплинути на дію РЕКAMBYS або інших лікарських засобів:
- карбамазепін, окскарбазепін, фенобарбітал, фенітоїн (лікарські засоби для лікування епілепсії та профілактики конвульсій)
- ріфабутин, рифампіцин, рифапентин (лікарські засоби для лікування бактеріальних інфекцій, таких як туберкульоз)
- дексаметазон (кортикостероїд, який використовується для лікування різних патологій, таких як запалення та алергічні реакції) у вигляді циклу лікування перорально або ін'єкційно
- продукти, які містять траву Св. Івана або гіперикум (Hypericum perforatum, лікарська рослина, яка використовується для лікування депресії).
Якщо ви приймаєте будь-який з цих лікарських засобів, проконсультуйтеся з вашим лікарем про альтернативи.
Попередження та застереження
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як почати використовувати РЕКAMBYS.
РЕКAMBYS не лікує інфекцію ВІЛ. Він є частиною лікування для зменшення кількості вірусу у крові. Під час використання цього лікарського засобу ви все ще можете передавати ВІЛ іншим людям, хоча ризик зменшується завдяки ефективному антивірусному лікуванню. Проконсультуйтеся з вашим лікарем про заходи, які потрібно вжити для того, щоб не інфікувати інших людей.
Повідомте вашому лікареві про вашу ситуацію
Перегляньте наступні пункти та повідомте вашому лікареві, якщо ви знаходитесь в одному з наступних випадків.
- Ви повинні відвідувати всі заплановані візити для ін'єкцій, не пропускайте жодного візиту, це дуже важливо для успіху вашого лікування. Якщо ви не можете відвідати запланований візит, повідомте вашому лікареві якнайшвидше.
- Повідомте вашому лікареві, якщо у вас є або був раніше гепатит, включно з гепатитом Б або гепатитом С, або ниркова хвороба. Ваш лікар, ймовірно, перевірить, як добре функціонують ваша печінка та нирки, щоб вирішити, чи можете ви використовувати РЕКAMBYS. Див. ознаки пошкодження печінки в розділі 4 цього опису «Побічні ефекти».
- Повідомте вашому лікареві негайно, якщо ви спостерігаєте будь-які симптоми інфекції(наприклад, гарячка, озноб, потіння). У деяких пацієнтів з ВІЛ може виникнути запалення через інфекції, які існували раніше, одразу після початку лікування проти ВІЛ. Говорять, що ці симптоми виникають через покращення імунної відповіді організму, яка дозволяє йому боротися з інфекціями, які існували раніше, але не мали явних симптомів.
- Повідомте вашому лікареві негайно, якщо ви спостерігаєте будь-які симптоми, наприклад, м'язову слабкість, слабкість, яка починається в руках і ногах і поширюється на тулуб, серцебиття, тремор або гіперактивність. Це відбувається через аутоімунні розлади (стані, при яких імунна система атакує здорові тканини організму помилково) після початку лікування проти ВІЛ. Аутоімунні розлади можуть виникнути через багато місяців після початку лікування.
- Повідомте вашому лікареві, якщо ви приймаєте будь-який лікарський засіб, який може викликати нерегулярний серцебиття, потенційно смертельне (торсаде де пойнтес).
Реакції на ін'єкції
Деякі люди 경험ували симптоми реакцій після ін'єкції через кілька хвилин після отримання ін'єкції рілпівіріни. Більшість симптомів зникли через кілька хвилин після ін'єкції. Симптоми реакцій після ін'єкції можуть включати: труднощі з диханням, м'язові спазми, потіння, оніміння рота, відчуття тривоги, відчуття жару, відчуття головокружіння або того, що ви можете потеряти свідомість (або знепритомніти) і зміни артеріального тиску. Повідомте вашому лікареві, якщо ви 경험уєте ці симптоми після отримання ін'єкцій.
Важливо відвідувати заплановані візити
Важливо, щоб ви відвідували заплановані візитидля отримання РЕКAMBYS для контролю інфекції ВІЛ і для того, щоб не допустити погіршення захворювання. Не пропускайте жодного візиту, це дуже важливо для успіху вашого лікування. Якщо ви не можете відвідати запланований візит, повідомте вашому лікареві якнайшвидше. Повідомте вашому лікареві, якщо ви думаєте припинити лікування. Якщо застосування РЕКAMBYS відкладається, або якщо ви припиняєте отримання РЕКAMBYS, вам потрібно буде приймати інші лікарські засоби для лікування інфекції ВІЛ і зменшення ризику того, що вірус стане резистентним, оскільки рівні лікарського засобу в вашому організмі будуть слишком низькими для лікування інфекції ВІЛ.
Діти
РЕКAMBYS не призначений для використання у дітей та підлітків молодше 18 років, оскільки він не був вивчений у цих пацієнтів.
Інші лікарські засоби та РЕКAMBYS
Повідомте вашому лікареві, якщо ви приймаєте, недавно приймали або можете приймати будь-який інший лікарський засіб. Деякі лікарські засоби можуть вплинути на рівень РЕКAMBYS у крові, якщо ви приймаєте їх під час лікування РЕКAMBYS, або РЕКAMBYS може вплинути на ефективність інших лікарських засобів.
Не використовувати РЕКAMBYS, якщо ви приймаєте будь-який з наступних лікарських засобів, оскільки вони можуть вплинути на дію РЕКAMBYS або інших лікарських засобів:
- карбамазепін, окскарбазепін, фенобарбітал, фенітоїн (лікарські засоби для лікування епілепсії та профілактики конвульсій)
- ріфабутин, рифампіцин, рифапентин (лікарські засоби для лікування бактеріальних інфекцій, таких як туберкульоз)
- дексаметазон (кортикостероїд, який використовується для лікування різних патологій, таких як запалення та алергічні реакції) у вигляді циклу лікування перорально або ін'єкційно
- продукти, які містять траву Св. Івана або гіперикум (Hypericum perforatum, лікарська рослина, яка використовується для лікування депресії).
Якщо ви приймаєте будь-який з цих лікарських засобів, проконсультуйтеся з вашим лікарем про альтернативи.
Вплив РЕКAMBYS або інших лікарських засобів може змінитися, якщо ви використовуєте РЕКAMBYS з будь-яким з наступних лікарських засобів:
- кларитроміцин, еритроміцин (антібіотики)
- метадон (який використовується для лікування синдрому абстиненції та залежності)
Вагітність та лактація
Повідомте вашому лікареві негайно, якщо ви вагітні або плануєте вагітність. Ваш лікар оцінить користь і ризик для вас і вашої дитини від використання РЕКAMBYS під час вагітності. Якщо ви плануєте вагітність, проконсультуйтеся з вашим лікарем раніше, оскільки рілпівіріна може залишатися в організмі до 4 років після останньої ін'єкції РЕКAMBYS.
Жінки з ВІЛ не повинні годувати грудьми, оскільки ВІЛ може передаватися через грудне молоко та інфікувати дитину.
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як приймати будь-який лікарський засіб.
Водіння автомобіля та використання машин
Деякі пацієнти можуть відчувати втому, головокружіння або сонливість під час лікування РЕКAMBYS. Не водьте автомобіль та не використовуйте машини, якщо ви відчуваєте будь-який з цих побічних ефектів.
Важлива інформація про деякі компоненти РЕКAMBYS
Цей лікарський засіб містить менше 1 ммоль натрію (23 мг) на 3 мл ін'єкції; тобто, він практично не містить натрію.
3. Як застосовується РЕКAMBYS
Медсестра або лікар введуть РЕКAMBYS у вигляді ін'єкції в м'яз гомілки (ін'єкція в м'яз).
Ін'єкція буде введена один раз на місяць або один раз на два місяці, разом з іншим лікарським засобом для ін'єкцій, який називається каботегравіром. Ваш лікар пояснить, з якою частотою вам буде введено лікарський засіб.
Перед тим, як почати лікування РЕКAMBYS, ваш лікар призначить вам лікування перорально таблетками рілпівіріни та каботегравіру протягом місяця. Це називається початкова пероральна доза;прийом таблеток перед ін'єкціями РЕКAMBYS та каботегравіру дозволить вашому лікареві перевірити, чи підходять ці лікарські засоби вам.
Якщо вам буде введено РЕКAMBYS один раз на місяць, ваше лікування буде наступним:
Коли | |||
Лікарський засіб | Місяць 1 (не менше 28 днів) | Місяць 2 (після місяця таблеток) | Від місяця 3 |
Рілпівіріна | Таблетка 25 мг один раз на день | Ін'єкція 900 мг | Ін'єкція 600 мг кожний місяць |
Каботегравір | Таблетка 30 мг один раз на день | Ін'єкція 600 мг | Ін'єкція 400 мг кожний місяць |
Якщо вам буде введено РЕКAMBYS кожні два місяці, ваше лікування буде наступним:
Коли | |||
Лікарський засіб | Місяць 1 (не менше 28 днів) | Місяць 2 (після місяця таблеток) і місяць 3 | Від місяця 5 |
Рілпівіріна | Таблетка 25 мг, один раз на день | Ін'єкція 900 мг | Ін'єкція 900 мг кожні два місяці |
Каботегравір | Таблетка 30 мг, один раз на день | Ін'єкція 600 мг | Ін'єкція 600 мг кожні два місяці |
Якщо ви пропустите ін'єкцію РЕКAMBYS
Важливо, щоб ви регулярно відвідували заплановані візити для отримання ін'єкції. Якщо ви не можете відвідати візит, негайно зв'яжіться з вашим лікарем, щоб призначити інший візит.
Повідомте вашому лікареві, якщо ви думаєте, що не зможете отримати ін'єкцію РЕКAMBYS у призначений день. Ваш лікар може порекомендувати вам приймати таблетки до тих пір, поки не зможете отримати ін'єкцію РЕКAMBYS знову.
Якщо вам буде введено надто багато РЕКAMBYS
Лікар або медсестра введуть вам цей лікарський засіб, тому малоймовірно, що вам буде введено надто багато. Якщо ви турбуєтесь, повідомте лікареві або медсестрі.
Не припиняйте використання РЕКAMBYS без поради вашого лікаря.
Використовуйте РЕКAMBYS весь час, поки ваш лікар не порадить вам інакше. Не переривайте лікування, якщо тільки ваш лікар не порадить вам це зробити.
Після припинення лікування можуть залишитися низькі рівні рілпівіріни (активної речовини РЕКAMBYS) у вашому організмі до 4 років. Однак після останньої ін'єкції РЕКAMBYS низькі рівні рілпівіріни, які залишилися, не будуть достатніми для боротьби з вірусом, і він може стати резистентним. Для того, щоб контролювати інфекцію ВІЛ-1 і уникнути того, що вірус стане резистентним, вам потрібно буде почати інше лікування проти ВІЛ у день, коли було призначено наступну ін'єкцію РЕКAMBYS.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
Нижче ви знайдете список побічних ефектів, які були описані при використанні РЕКAMBYS та каботегравіру для ін'єкцій.
Дуже часті побічні ефекти (впливають на щонайменше 1 з 10 осіб)
- головний біль
- реакції в місці ін'єкції - зазвичай вони легкі або помірні та їхня частота зменшується з часом. Їхні симптоми можуть включати:
о дуже часті: біль і дискомфорт, маси або твердіння
о часті: червоність, свербіж, набряк, синяки, тепло або зміна кольору.
о рідкі: оніміння, легке кровотечение, утворення абсцесу (накопичення гною) або целюліту (з відчуттям тепла, набряку або червоності).
- чуття жару/гарячки (пірексія)
Часті побічні ефекти (впливають на менше 1 з 10 осіб)
- депресія
- тривога
- анормальні сни
- труднощі з сном (безсоння)
- головокружіння
- чуття нудоти (нудота)
- вомітування
- біль у животі (біль в животі)
- гази (вздування)
- діарея
- виразка
- біль у м'язах (міалгія)
- втома (фатига)
- чуття слабкості (астенія)
- загальне нездужання
- збільшення ваги
Рідкі побічні ефекти (впливають на менше 1 з 100 осіб)
- сонливість (сомноленція)
- чуття головокружіння під час або після ін'єкції. Це може викликати знепритомнення.
- пошкодження печінки (її ознаки можуть включати жовтушність шкіри та білкової частини очей, втрату апетиту, свербіж, біль при пальпації живота, світлий кал або сеча ненормально темного кольору).
- зміни в аналітичних показниках функції печінки (збільшення трансаміназ)
- збільшення білірубіну(речовини, яку виробляє печінка) у крові.
Інші побічні ефекти
- Тяжкий біль у животі, викликаний запаленням підшлункової залози (панкреатит).
Наступні побічні ефекти, які можуть виникнути при застосуванні рілпівіріни у вигляді таблеток, також можуть виникнути при застосуванні РЕКAMBYS для ін'єкцій:
Дуже часті побічні ефекти
- збільшення рівня холестерину та/або амілази підшлункової залози у крові
Часті побічні ефекти (впливають на менше 1 з 10 осіб)
- втрата апетиту
- розлади сну
- депресивний стан
- дискомфорт у животі
- сухість у роті
- низький рівень лейкоцитів та/або тромбоцитів, зниження гемоглобіну у крові, збільшення тригліцеридів та/або ліпази у крові
Рідкі побічні ефекти (впливають на менше 1 з 100 осіб)
- ознаки або симптоми запалення чи інфекції, наприклад гарячка, озноб, потіння (синдром імунної реконструкції, для більшої інформації див. розділ 2)
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не вказані в цьому описі. Ви також можете повідомити про них безпосередньо через національну систему повідомлень, включену до додатку V. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Збереження РЕКAMBYS
Тримайте його поза зоною видимості та досягнення дітей.
Не використовувати цей лікарський засіб після закінчення терміну придатності, який вказаний на упаковці після «CAD». Термін придатності - останній день місяця, який вказаний.
Тримайте в холодильнику (між 2°C та 8°C). Не заморожуйте.
Лікарські засоби не повинні викидатися у водопровідні труби чи сміття. Спитайте вашого фармацевта, як позбутися упаковок та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад REKAMBYS
- Активний інгредієнт - рілпівірин. Кожна флакон містить 900 мг рілпівіріну.
- Допоміжні речовини - полоксамер 338, цитринова кислота моногідрат, глюкоза моногідрат, дигідрогенфосфат натрію моногідрат, гідроксид натрію для регулювання pH і забезпечення ізотонічності, вода для ін'єкцій.
Вигляд продукту та вміст упаковки
Ін'єкційна суспензія з тривалим вивільненням. REKAMBYS випускається у скляному флаконі. Упаковка також містить 1 шприц, 1 адаптер флакону та 1 голку для ін'єкції.
Власник дозволу на маркетинг
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Бельгія
Виробник
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Бельгія
Для отримання додаткової інформації про цей препарат зверніться до місцевого представника власника дозволу на маркетинг:
Бельгія/Бельгія/Бельгія ViiV Healthcare srl/bv Тел./Телефон: + 32 (0) 10 85 65 00 | Литва Уаб "Джонсон та Джонсон" Телефон: +370 5 278 68 88 |
Україна «Джонсон та Джонсон Україна» ТОВ Телефон: +380 44 490 90 00 | Люксембург/Люксембург ViiV Healthcare srl/bv Бельгія/Бельгія Тел./Телефон: + 32 (0) 10 85 65 00 |
Чехія Janssen-Cilag s.r.o. Телефон: +420 227 012 227 | Угорщина Janssen-Cilag Kft. Телефон: +36 1 884 2858 |
Данія Janssen-Cilag A/S Телефон: +45 4594 8282 | Мальта AM MANGION LTD. Телефон: +356 2397 6000 |
Німеччина ViiV Healthcare GmbH Телефон: + 49 (0)89 203 0038-10 | Нідерланди ViiV Healthcare BV Телефон: + 31 (0) 33 2081199 |
Естонія Уаб "Джонсон та Джонсон" Естонський філіал Телефон: +372 617 7410 | Норвегія Janssen-Cilag AS Телефон: +47 24 12 65 00 |
Греція Janssen-Cilag Φαρμακευτικη Α.Ε.Β.Ε. Телефон: +30 210 80 90 000 | Австрія Janssen-Cilag Pharma GmbH Телефон: +43 1 610 300 |
Іспанія Laboratorios ViiV Healthcare, S.L. Телефон: + 34 900 923 501 | Польща Janssen-Cilag Polska Sp. z o.o. Телефон: +48 22 237 60 00 |
Франція ViiV Healthcare SAS Телефон: + 33 (0)1 39 17 69 69 | Португалія VIIVHIV HEALTHCARE, UNIPESSOAL, LDA Телефон: + 351 21 094 08 01 |
Хорватія Johnson & Johnson S.E. d.o.o. Телефон: +385 1 6610 700 | Румунія Johnson & Johnson România SRL Телефон: +40 21 207 1800 |
Ірландія Janssen Sciences Ireland UC Телефон: +353 1 800 709 122 | Словенія Johnson & Johnson d.o.o. Телефон: +386 1 401 18 00 |
Ісландія Janssen-Cilag AB c/o Vistor hf. Телефон: +354 535 7000 | Словаччина Johnson & Johnson s.r.o. Телефон: +421 232 408 400 |
Італія ViiV Healthcare S.r.l Телефон: +39 045 7741600 | Фінляндія Janssen-Cilag Oy Телефон: +358 207 531 300 |
Кіпр Βαρνάβας Χατζηπαναγής Λτδ Телефон: +357 22 207 700 | Швеція Janssen-Cilag AB Телефон: +46 8 626 50 00 |
Латвія Уаб "Джонсон та Джонсон" філіал Латвія Телефон: +371 678 93561 | Велика Британія ViiV Healthcare UK Limited Телефон: + 44 (0)800 221441 |
Дата останнього перегляду цього листка: {MM/РРРР}.
Детальна інформація про цей препарат доступна на сайті Європейського агентства з лікарських засобів: http://www.ema.europa.eu/.
Ця інформація призначена лише для лікарів або медичних працівників, які повинні читати її разом з повною інформацією про препарат (Резюме характеристик продукту).
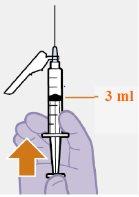
Інструкції з використання REKAMBYS 3 мл ін'єкційного:
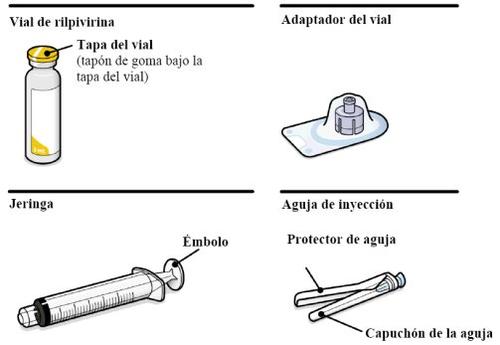
Резюме Повна доза складається з двох ін'єкцій: 3 мл каботегравіру та 3 мл рілпівіріну. Каботегравір та рілпівірин представлені у вигляді суспензій, які не потребують розбавлення або реконституції. Відповідні кроки для підготовки обидвох препаратів однакові. Каботегравір та рілпівірин призначені лише для інтримускульної ін'єкції. Обидві ін'єкції повинні бути введені в сідничну область. Порядок введення не має значення. Примітка:Рекомендується вводити в вентиросідничну область. | |
Інформація про зберігання | |
Незаморожувати. | |
| |
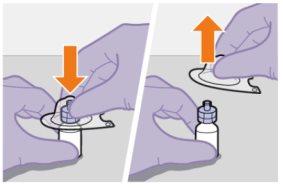
Ваше упаковання містить | |
Зверніть увагу на стан пацієнта та використовуйте свій медичний досвід для вибору голки відповідної довжини. | |
Вам також знадобиться | |
| |
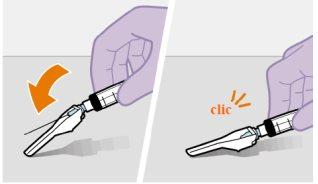
Підготовка | |
| |
|
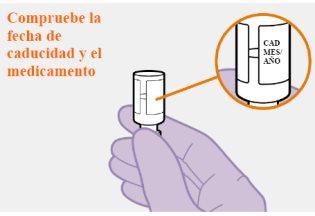
Не використовуйте, якщо термін придатності закінчився. |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
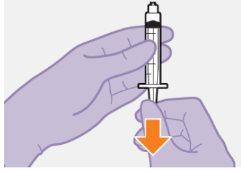
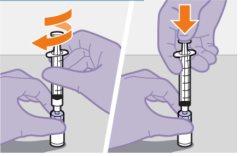
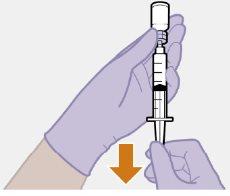
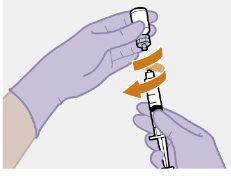
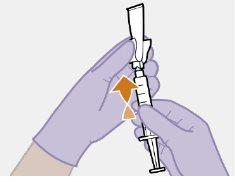
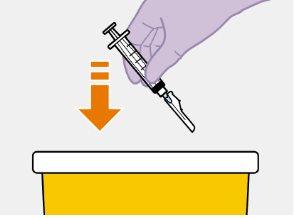
Примітка: Тримайте шприц вверх, щоб уникнути крапання. Перевірте, чи має суспензія однорідний вигляд та білий колір. |
| |
|
|
Ін'єкція | |
| |
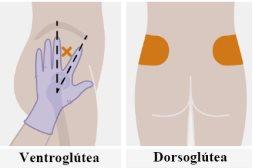
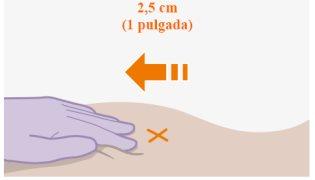
| Ін'єкції повинні бути введені в сідничну область. Виберіть одну з наступних зон для ін'єкції:
Примітка: Лише для інтримускульної ін'єкції в сідничну область. Невводьте внутрішньовенно. |
| |
|
|
| |
|
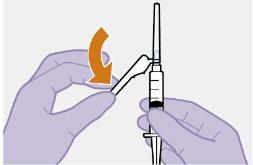
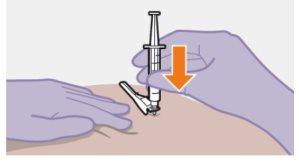
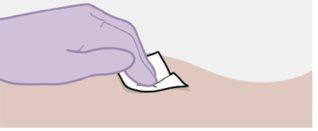
Примітка: Очистіть місце ін'єкції ватним тампоном, змоченим спиртом. Дайте шкірі висохнути на повітрі, перш ніж продовжити. |
| |
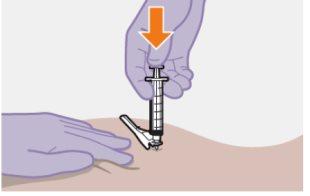
| Використовуйте техніку ін'єкції за схемою "з", щоб мінімізувати можливість виходу препарату з місця ін'єкції.
|
| |
|
|
| |
|
|
| |
|
Немасажуйте область. |
| |
|
|
Після ін'єкції | |
| |
|
|
Повторіть процес для другого препарату | |
| Якщо ви ще не ввели обидва препарати, дотримуйтесь кроків для підготовки та ін'єкції каботегравіру, який має свої власні інструкції з використання. |
Питання та відповіді | |
Найкраще вводити препарат негайно після досягнення кімнатної температури. Однак флакон можна залишити в упаковці при кімнатній температурі (максимальна температура 25°C) протягом максимально 6 годин.
Найкраще вводити препарат (при кімнатній температурі) якомога скоріше після видалення з флакону. Однак препарат можна залишити в шприці протягом максимально 2 годин перед введенням. Якщо ви перевищили 2 години, викиньте препарат, шприц та голку.
Введення 1 мл повітря у флакон полегшує видалення дози шприцем. Якщо не вводити повітря, частина рідини може повернутися до флакону випадково, залишивши недостатню кількість у шприці.
Ні, порядок не має значення.
Найкраще дати флакону досягнути кімнатної температури природним чином. Однак можна використовувати тепло рук, щоб прискорити процес, але переконайтеся, що флакон не перевищує 25°C Не використовуйте жоден інший метод для нагрівання.
Рекомендується введення у вентиросідничну область, у середній м'яз сідниць, оскільки це область, в якій немає важливих нервів чи судин поблизу. Якщо медичний працівник цього бажає, також прийнятне введення у дорсосідничну область, у великий м'яз сідниць. Ін'єкцію не слід вводити в жодній іншій області. |
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до РЕКАМБИС 900 МГ СУСПЕНЗІЯ ДЛЯ ІН'ЄКЦІЙ ПРОЛОНГОВАНОЇ ДІЇФорма випуску: ТАБЛЕТКА, 25 мг рилпівиринуДіючі речовини: rilpivirineВиробник: Janssen-Cilag International N.VПотрібен рецептФорма випуску: ТАБЛЕТКА, 600 мгДіючі речовини: efavirenzВиробник: Aurovitas Spain, S.A.U.Потрібен рецепт
Лікарі онлайн щодо РЕКАМБИС 900 МГ СУСПЕНЗІЯ ДЛЯ ІН'ЄКЦІЙ ПРОЛОНГОВАНОЇ ДІЇ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на РЕКАМБИС 900 МГ СУСПЕНЗІЯ ДЛЯ ІН'ЄКЦІЙ ПРОЛОНГОВАНОЇ ДІЇ – за рішенням лікаря та згідно з місцевими правилами.