OLIMEL N9E EMULSION FOR PERFUSION

How to use OLIMEL N9E EMULSION FOR PERFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
Olimel N9E emulsion for infusion
Read the entire leaflet carefully before this medicine is administered to you, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or nurse.
- If you experience side effects, consult your doctor or nurse, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet
- What Olimel N9E is and what it is used for
- What you need to know before Olimel N9E is administered to you
- How Olimel N9E will be administered to you
- Possible side effects
- Storage of Olimel N9E
- Contents of the pack and further information
1. What OLIMEL N9E is and what it is used for
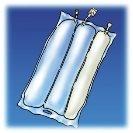
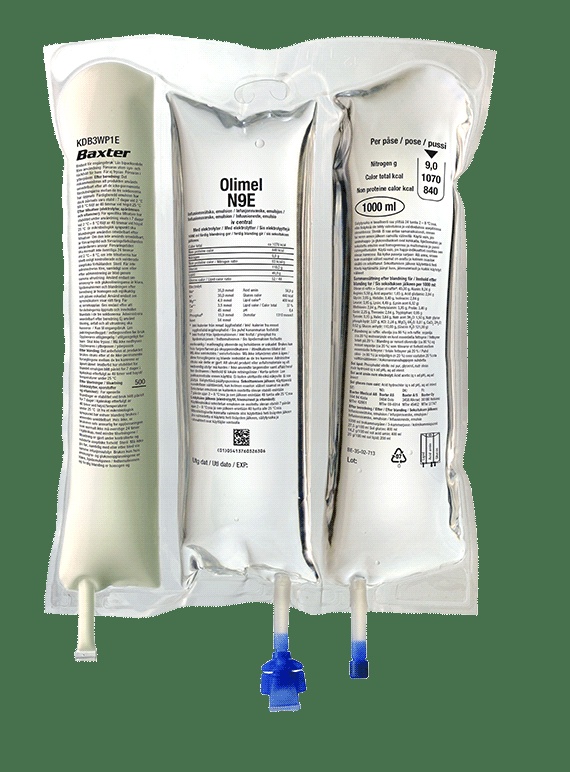
Olimel is an emulsion for infusion. It comes in a bag with 3 chambers.
One chamber contains a glucose solution with calcium, the second contains a lipid emulsion, and the third contains an amino acid solution with other electrolytes.
Olimel is used to feed adults and children over 2 years of age through a tube in a vein when normal feeding by mouth is not possible.
Olimel should only be used under medical supervision.
2. What you need to know before Olimel N9E is administered to you
Olimel N9E must not be administered:
- To premature neonates, babies, and children under 2 years of age.
- If you are hypersensitive (allergic) to egg proteins, soybean proteins, peanut proteins, corn/maize products (see also the "Warnings and precautions" section below) or to any of the other components of this medicine (listed in section 6).
- If your body has problems using certain amino acids.
- If you have a particularly high level of fats in the blood.
- If you have hyperglycemia (too much sugar in the blood).
- If you have an abnormally high level of some electrolyte (sodium, potassium, magnesium, calcium, and/or phosphorus) in the blood.
In all cases, your doctor will decide whether you should be given this medicine based on factors such as your age, weight, and clinical condition, along with the results of all tests performed.
Warnings and precautions
Consult your doctor or nurse before Olimel is administered to you.
Too rapid administration of total parenteral nutrition (TPN) solutions may cause injury or death.
The infusion should be stopped immediately if any abnormal signs or symptoms of an allergic reaction (such as sweating, fever, chills, headache, skin rash, or difficulty breathing) develop. This medicine contains soybean oil and egg phospholipids. Soybean and egg proteins may cause hypersensitivity reactions. Cross-allergic reactions have been observed between soybean and peanut proteins.
Olimel contains glucose derived from corn, which may cause hypersensitivity reactions if you are allergic to corn or corn products (see "Olimel N9E must not be administered" section above).
Difficulty breathing could also be a sign that small particles have formed in the lungs, blocking blood vessels (pulmonary vascular precipitates). If you experience any difficulty breathing, inform your doctor or nurse. They will decide on the course of action to take.
The antibiotic called ceftriaxone must not be mixed or administered simultaneously with solutions containing calcium (including Olimel) given by drip into a vein.
These medicines must not be administered together, even through different lines or infusion sites.
However, Olimel and ceftriaxone may be administered sequentially, one after the other, if infusion lines are used at different points, or if the infusion lines are replaced or thoroughly flushed with physiological saline solution between infusions to avoid the formation of precipitates (formation of ceftriaxone and calcium salt particles).
Certain medicines and diseases may increase the risk of developing infections or sepsis (bacteria in the blood). There is a risk of infection or sepsis, especially when a tube (intravenous catheter) is placed in a vein. Your doctor will closely monitor you for signs of infection.
Patients who require parenteral nutrition (administration of nutrients through a tube inserted into a vein) may be more prone to infections due to their medical condition. The use of "aseptic techniques" (germ-free) when placing and maintaining the catheter and preparing the nutritional formula (TPN) can reduce the risk of infection.
If you are severely malnourished to the point of requiring intravenous feeding, your doctor will start treatment slowly. Additionally, you will be monitored to avoid sudden changes in your fluid, vitamin, electrolyte, and mineral levels.
Before starting the infusion, any metabolic disorders and water and salt balance in your body should be corrected. Your doctor will monitor your condition while you are being administered this medicine and may change the dose or add other nutrients, such as vitamins, electrolytes, and trace elements, if deemed necessary.
Cases of liver disorders, including problems with bile elimination (cholestasis), fat storage (hepatic steatosis), fibrosis, which may lead to liver failure, as well as cholecystitis and cholelithiasis, have been reported in patients receiving intravenous nutritional treatment. It is believed that the cause of these disorders is due to multiple factors and may differ between patients. If you experience symptoms such as nausea, vomiting, abdominal pain, yellowing of the skin or eyes, consult your doctor to identify possible causative and contributing factors and possible therapeutic and preventive measures.
Your doctor should be aware if you have:
- any severe kidney problem. You should also inform your doctor if you are receiving dialysis treatment (artificial kidney) or if you have any other type of treatment to clean the blood.
- any severe liver problem.
- any blood coagulation problem.
- abnormal functioning of the adrenal glands (adrenal insufficiency). The adrenal glands are triangular in shape and are located above the kidneys.
- heart failure.
- lung disease.
- fluid accumulation in the body (hyperhydration).
- insufficient water in the body (dehydration).
- excess sugar in the blood (diabetes mellitus) without treatment for it.
- heart attack or shock due to sudden heart failure.
- severe metabolic acidosis (blood too acidic).
- generalized infection (septicemia).
- coma.
To check the effectiveness and safety of administration, your doctor will perform laboratory and clinical tests while you are being administered this medicine. If you are administered this medicine for several weeks, your blood will be regularly analyzed.
The decrease in the body's ability to eliminate the fats contained in this medicine may lead to a "fat overload syndrome" (see section 4 "Possible side effects").
If during the infusion you notice pain, burning, or swelling at the infusion site or leakage of the infusion, inform your doctor or nurse. The administration will be stopped immediately and restarted in another vein.
If your blood sugar levels rise too high, your doctor will adjust the rate of Olimel administration or provide you with medication to control blood sugar levels (insulin).
Olimel can only be administered through a tube (catheter) connected to a large vein in your chest (central vein).
Children and adolescents
If your child is under 18 years of age, special attention will be paid to the administration of the correct dose. Additional precautions will also be taken due to the greater sensitivity of children to the risk of infection. Supplementation with vitamins and trace elements is always necessary. Pediatric formulations should be used.
Use of Olimel with other medicines
Tell your doctor if you are taking or using, have recently taken or used, or might take or use any other medicine.
Simultaneous absorption of other medicines is generally not a contraindication. If you are taking other medicines, obtained with or without a prescription, you should consult your doctor in advance to check if they are compatible.
Inform your doctor if you are taking or receiving any of the following medicines:
- insulin.
- heparin.
Olimel must not be administered simultaneously with blood through the same infusion line.
Olimel contains calcium. It must not be administered together or through the same line with the antibiotic ceftriaxone, as particles may form. If the same device is used to administer these medicines sequentially, it must be thoroughly flushed.
Due to the risk of precipitation, Olimel must not be administered through the same infusion line or mixed with the antibiotic ampicillin or the antiepileptic fosphenytoin.
The olive and soybean oils present in Olimel contain vitamin K. This usually does not affect blood-thinning medicines (anticoagulants) such as coumarin. However, if you are taking anticoagulants, you should inform your doctor.
The lipids in this emulsion may interfere with the results of certain laboratory tests if the blood sample is taken before they have been eliminated from your bloodstream (usually after a period of 5 to 6 hours without receiving lipids).
Olimel contains potassium. Special care should be taken in patients who are taking diuretics, ACE inhibitors, angiotensin II receptor antagonists (medicines for high blood pressure), or immunosuppressants. These classes of medicines may increase potassium levels in the blood.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before this medicine is administered to you.
There is no adequate experience with the use of Olimel in pregnant or breastfeeding women. Olimel may be used during pregnancy and breastfeeding if necessary. Olimel should only be administered to pregnant or breastfeeding women after careful consideration.
Driving and using machines
Not applicable.
3. How Olimel N9E will be administered to you
Dose
Olimel should only be administered to adults and children over 2 years of age.
This is an emulsion for infusion, i.e., for administration through a tube (catheter) into a large vein in your chest.
Olimel should be at room temperature before use.
Olimel is for single use only.
Dose – Adults
Your doctor will determine an infusion rate based on your needs and clinical condition.
Administration may continue for as long as necessary, depending on your clinical condition.
Dose – Children over 2 years and adolescents
The doctor will decide on the dose and the duration of administration, based on age, weight, height, clinical condition, and the body's ability to break down and use the ingredients of OLIMEL.
If you have been administered too much Olimel N9E
If the dose administered is too high or the infusion is too rapid, the amino acid content may make your blood too acidic, and signs of hypervolemia (increased circulating blood volume) may occur. Your blood and urine glucose levels may increase, a hyperosmolar syndrome may develop, and the lipid content may increase your blood triglycerides. Administration of an infusion that is too rapid or a volume that is too high may cause nausea, vomiting, chills, headache, fever, sweating (hyperhidrosis), and electrolyte disturbances. In this case, the infusion should be stopped immediately.
In severe cases, your doctor may need to perform temporary renal dialysis to help your kidneys eliminate excess product.
To avoid these cases, your doctor will regularly monitor your condition and analyze your blood parameters.
If you have any further questions on the use of this product, ask your doctor.
In case of overdose or accidental ingestion, consult the Toxicological Information Service. Phone 915.620.420
4. Possible side effects
Like all medicines, this medicine may cause side effects, although not everybody gets them. If you notice that you do not feel as you did before, inform your doctor or nurse immediately.
The tests that your doctor will perform while you are taking this medicine should minimize the risk of side effects.
If any abnormal signs or symptoms of an allergic reaction, such as sweating, fever, chills, headache, skin rash, or difficulty breathing, develop, the infusion should be stopped immediately.
The following side effects have been reported with Olimel:
Frequency – Common: may affect up to 1 in 10 people
- Fast heart rate (tachycardia).
- Decreased appetite.
- Increased level of fats in the blood (hypertriglyceridemia).
- Abdominal pain.
- Diarrhea.
- Nausea.
- High blood pressure (hypertension).
Frequency - Unknown: cannot be estimated from the available data
- Hypersensitivity reactions, including sweating, fever, chills, headache, skin rash (erythematous, papular, pustular, macular, generalized rash), itching, flushing, difficulty breathing.
- Infusion site reactions, including pain, irritation, swelling/edema, redness (erythema)/heat, cell death (necrosis) or blisters/vesicles, inflammation, thickening, or constriction of the skin.
- Vomiting.
The following side effects have been reported with other similar products for parenteral nutrition:
Frequency - Very rare: may affect up to 1 in 10,000 people
- Reduced ability to eliminate lipids (fat overload syndrome) associated with a sudden and severe worsening of the patient's medical condition. The following symptoms of fat overload syndrome are usually reversible when the lipid emulsion infusion is stopped:
- Fever.
- Decreased red blood cell count, which may cause pale skin and weakness or difficulty breathing (anemia).
- Low white blood cell count, which may increase the risk of infection (leukopenia).
- Low platelet count, which may increase the risk of bruising and/or bleeding (thrombocytopenia).
- Coagulation disorders affecting the blood's ability to clot.
- High levels of fats in the blood (hyperlipidemia).
- Fat accumulation in the liver (hepatomegaly).
- Worsening of liver function.
- Central nervous system symptoms (e.g., coma).
Frequency - Unknown: cannot be estimated from the available data
- Allergic reactions.
- Bile elimination problems (cholestasis).
- Abnormal liver function blood tests.
- Increased liver size (hepatomegaly).
- Diseases associated with parenteral nutrition (see "Warnings and precautions" in section 2).
- Jaundice.
- Low platelet count (thrombocytopenia).
- Increased nitrogen levels in the blood (azotemia).
- Increased liver enzymes.
- Formation of small particles that can lead to blockage of blood vessels in the lungs (pulmonary vascular precipitates), resulting in pulmonary vascular embolism and difficulty breathing (respiratory distress).
Reporting of side effects:
If you experience any side effects, consult your doctor or nurse, even if they are side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Olimel N9E
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging and outer packaging (MM/YYYY). The expiry date is the last day of the month indicated.
Do not freeze.
Store in the overbag.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition ofOlimelN9E
The active ingredients of each bag of the reconstituted emulsion are a 14.2% L-amino acid solution (corresponding to 14.2 g/100 ml of alanine, arginine, glycine, histidine, isoleucine, leucine, lysine (as lysine acetate), methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine, aspartic acid, and glutamic acid) with electrolytes (sodium, potassium, magnesium, phosphate, acetate, chloride), a 20% lipid emulsion (corresponding to 20 g/100 ml of refined olive oil and refined soybean oil) and a 27.5% glucose solution (corresponding to 27.5 g/100 ml as glucose monohydrate) with calcium.
The other components are:
Lipid Emulsion Compartment | Amino Acid Solution Compartment | Glucose Solution Compartment |
Purified egg phospholipids, glycerol, sodium oleate, sodium hydroxide (for pH adjustment), water for injectable preparations | Glacial acetic acid (for pH adjustment), water for injectable preparations | Hydrochloric acid (for pH adjustment), water for injectable preparations |
Appearance ofOlimel N9Eand Container Contents
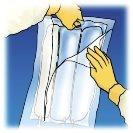
Olimel is an emulsion for infusion packaged in a 3-compartment bag. One compartment contains a lipid emulsion, another a solution of amino acids with electrolytes, and the third a solution of glucose with calcium. These compartments are separated by non-permanent seals. Before administration, the contents of the compartments must be mixed by rotating the bag from top to bottom until the seals are open.
Appearance before reconstitution:
- The amino acid and glucose solutions are clear, colorless, or slightly yellowish.
- The lipid emulsion is homogeneous and milky white.
Appearance after reconstitution: Homogeneous milky emulsion.
The three-compartment bag is a plastic bag with multiple layers. The material of the inner layer (contact) of the bag is designed to be compatible with the authorized components and additives.
To avoid contact with air oxygen, the bag is packaged in an outer bag that acts as an oxygen barrier, which contains a sachet with an oxygen absorber.
Container Sizes
1000 ml bag: 1 cardboard box with 6 bags
1500 ml bag: 1 cardboard box with 4 bags
2000 ml bag: 1 cardboard box with 4 bags
1 bag of 1000 ml, 1500 ml, and 2000 ml
Only some container sizes may be marketed.
Marketing Authorization Holder
Baxter S.L.
Pouet de Camilo 2, 46394 Ribarroja del Turia (Valencia)
Manufacturer
Baxter S.A., Boulevard René Branquart, 80, 7860 Lessines, Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
France, Portugal, Estonia, Poland, Lithuania, Bulgaria, Romania, Latvia, Czech Republic, Belgium, Spain, Slovakia, Netherlands, Luxembourg, Slovenia, Italy, Greece, Cyprus: OLIMEL N9E
In some countries, it is registered under different names as described below:
Austria: ZentroOLIMEL 5.7% mit Elektrolyten
Germany: Olimel 5.7% E
Denmark, Iceland, Sweden, Norway, Finland: Olimel N9E
United Kingdom, Ireland, and Malta: Triomel 9g/l nitrogen 1070 kcal/l with electrolytes
Hungary: Olimel 9 g/l nitrogén elektrolitokkal emulziós infúzió
Date of the last revision of this leafletApril 2020
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
This information is intended only for healthcare professionals
Pharmacotherapeutic group: solutions for parenteral nutrition / combinations
ATC code: B05 BA10.
- Qualitative and quantitative composition
Olimel is presented in the form of a 3-compartment bag. Each bag contains a glucose solution with calcium, a lipid emulsion, and a solution of amino acids with other electrolytes.
Content per bag | |||
1000 ml | 1500 ml | 2000 ml | |
27.5% glucose solution (corresponding to 27.5 g/100 ml) | 400 ml | 600 ml | 800 ml |
14.2% amino acid solution (corresponding to 14.2 g/100 ml) | 400 ml | 600 ml | 800 ml |
20% lipid emulsion (corresponding to 20 g/100 ml) | 200 ml | 300 ml | 400 ml |
After mixing the contents of the 3 compartments, the composition of the reconstituted emulsion is indicated in the following table for each bag size.
Active Ingredients | 1000 ml | 1500 ml | 2000 ml |
Refined olive oil + refined soybean oil Alanine Arginine Aspartic acid Glutamic acid Glycine Histidine Isoleucine Leucine Lysine (equivalent to Lysine acetate) Methionine Phenylalanine Proline Serine Threonine Tryptophan Tyrosine Valine Sodium acetate trihydrate Sodium glycerophosphate hydrate Potassium chloride Magnesium chloride hexahydrate Calcium chloride dihydrate Glucose (equivalent to glucose monohydrate) | 40.00 g 8.24 g 5.58 g 1.65 g 2.84 g 3.95 g 3.40 g 2.84 g 3.95 g 4.48 g (6.32 g) 2.84 g 3.95 g 3.40 g 2.25 g 2.84 g 0.95 g 0.15 g 3.64 g 1.50 g 3.67 g 2.24 g 0.81 g 0.52 g 110.00 g (121.00 g) | 60.00 g 12.36 g 8.37 g 2.47 g 4.27 g 5.92 g 5.09 g 4.27 g 5.92 g 6.72 g (9.48 g) 4.27 g 5.92 g 5.09 g 3.37 g 4.27 g 1.42 g 0.22 g 5.47 g 2.24 g 5.51 g 3.35 g 1.22 g 0.77 g 165.00 g (181.50 g) | 80.00 g 16.48 g 11.16 g 3.30 g 5.69 g 7.90 g 6.79 g 5.69 g 7.90 g 8.96 g (12.64 g) 5.69 g 7.90 g 6.79 g 4.50 g 5.69 g 1.90 g 0.30 g 7.29 g 2.99 g 7.34 g 4.47 g 1.62 g 1.03 g 220.00 g (242.00 g) |
(a) Mixture of refined olive oil (approximately 80%) and refined soybean oil (approximately 20%) corresponding to a ratio of essential fatty acids / total fatty acids of 20%
The excipients are:
Lipid Emulsion Compartment | Amino Acid Solution Compartment with Electrolytes | Glucose Solution Compartment with Calcium |
Purified egg phospholipids, glycerol, sodium oleate, sodium hydroxide (for pH adjustment), water for injectable preparations | Glacial acetic acid (for pH adjustment), water for injectable preparations | Hydrochloric acid (for pH adjustment), water for injectable preparations |
The reconstituted emulsion provides the following for each bag size:
1000 ml | 1500 ml | 2000 ml | |
Lipids | 40 g | 60 g | 80 g |
Amino acids | 56.9 g | 85.4 g | 113.9 g |
Nitrogen | 9.0 g | 13.5 g | 18.0 g |
Glucose | 110.0 g | 165.0 g | 220.0 g |
Energy: | |||
Approximate total calories | 1070 kcal | 1600 kcal | 2140 kcal |
Non-protein calories | 840 kcal | 1260 kcal | 1680 kcal |
Glucose calories | 440 kcal | 660 kcal | 880 kcal |
Lipid calories (a) | 400 kcal | 600 kcal | 800 kcal |
Non-protein calories / nitrogen ratio | 93 kcal/g | 93 kcal/g | 93 kcal/g |
Glucose / lipid calories ratio | 52/48 | 52/48 | 52/48 |
Lipid calories / total calories | 37% | 37% | 37% |
Electrolytes: | |||
Sodium | 35.0 mmol | 52.5 mmol | 70.0 mmol |
Potassium | 30.0 mmol | 45.0 mmol | 60.0 mmol |
Magnesium | 4.0 mmol | 6.0 mmol | 8.0 mmol |
Calcium | 3.5 mmol | 5.3 mmol | 7.0 mmol |
Phosphate (b) | 15.0 mmol | 22.5 mmol | 30.0 mmol |
Acetate | 54 mmol | 80 mmol | 107 mmol |
Chloride | 45 mmol | 68 mmol | 90 mmol |
pH | 6.4 | 6.4 | 6.4 |
Osmolality | 1310 mOsm/l | 1310 mOsm/l | 1310 mOsm/l |
a Includes calories from purified egg phospholipids
b Includes phosphate provided by the lipid emulsion
- Dosage and Administration
Dosage
Olimel is not recommended for use in children under 2 years of age, as neither the composition nor the volume is suitable (see sections 4.4, 5.1, and 5.2 of the Technical Sheet).
The maximum daily dose mentioned below should not be exceeded. Due to the invariant composition of the multicompartimental bag, the ability to simultaneously meet all the patient's nutritional needs may not be possible. There may be clinical situations where the patient requires amounts of nutrients that vary from the composition of the bag. In this situation, any adjustment of volume (dose) must take into account the resulting effect on the dosing of the other nutrients of OLIMEL.
In adults
The dose depends on the patient's energy expenditure, clinical status, body weight, and ability to metabolize the components of Olimel, as well as any additional energy or proteins administered orally or enterally. Therefore, the appropriate bag size should be chosen.
The average daily needs are:
- 0.16 to 0.35 g of nitrogen/kg of body weight (1 to 2 g of amino acids/kg), depending on the patient's nutritional status and level of catabolic stress.
- 20 to 40 kcal/kg.
- 20 to 40 ml of fluid/kg, or 1 to 1.5 ml per kcal expended.
For Olimel, the maximum daily dose is defined by the intake of amino acids, 35 ml/kg, corresponding to 2.0 g/kg of amino acids, 3.9 g/kg of glucose, 1.4 g/kg of lipids, 1.2 mmol/kg of sodium, and 1.1 mmol/kg of potassium. For a 70 kg patient, this would be equivalent to 2450 ml of Olimel per day, which would provide 140 g of amino acids, 270 g of glucose, and 98 g of lipids, i.e., 2058 non-protein kcal and 2622 total kcal.
Normally, the administration rate should be gradually increased during the first hour and then adjusted according to the dose being administered, daily volume intake, and duration of infusion.
For Olimel, the maximum infusion rate is 1.8 ml/kg/hour, corresponding to 0.10 g/kg/hour of amino acids, 0.19 g/kg/hour of glucose, and 0.07 g/kg/hour of lipids.
In children over 2 years and adolescents
No studies have been conducted in the pediatric population.
The dose depends on the patient's energy expenditure, clinical status, weight, and ability to metabolize the components of Olimel, as well as any additional energy and proteins administered orally or enterally. Therefore, the appropriate bag size should be chosen.
Additionally, the daily needs for fluid, nitrogen, and energy decrease continuously with age: Two age groups are considered, one between 2 and 11 years, and another between 12 and 18 years.
For Olimel N9E, in the 2 to 11-year-old group, the magnesium concentration is the limiting factor for the daily dose, while the glucose concentration is the limiting factor for the hourly rate. In the 12 to 18-year-old group, the limiting factors for the daily dose are the amino acid and magnesium concentrations, and the amino acid concentration for the hourly rate. The resulting intakes are as follows:
Constituent | 2 to 11 years | 12 to 18 years | ||
Recommended (a) | OLIMEL N9E Vol. Max | Recommended (a) | OLIMEL N9E Vol. Max | |
Maximum daily dose | ||||
Fluid (ml/kg/day) | 60 – 120 | 25 | 50 – 80 | 35 |
Amino acids (g/kg/day) | 1 – 2 (up to 2.5) | 1.4 | 1 – 2 | 2.0 |
Glucose (g/kg/day) | 1.4 – 8.6 | 2.8 | 0.7 – 5.8 | 3.9 |
Lipids (g/kg/day) | 0.5 – 3 | 1.0 | 0.5 – 2 (up to 3) | 1.4 |
Total energy (kcal/kg/day) | 30 – 75 | 26.8 | 20 – 55 | 37.5 |
Maximum hourly rate | ||||
OLIMEL N9E (ml/kg/h) | 3.3 | 2.1 | ||
Amino acids (g/kg/h) | 0.20 | 0.19 | 0.12 | 0.12 |
Glucose (g/kg/h) | 0.36 | 0.36 | 0.24 | 0.23 |
Lipids (g/kg/h) | 0.13 | 0.13 | 0.13 | 0.08 |
a: Recommended values in the 2018 ESPGHAN/ESPEN/ESPR Guidelines
Normally, the administration rate should be gradually increased during the first hour and then adjusted according to the dose being administered, daily volume intake, and duration of infusion.
In general, in the case of small children, it is recommended to start the infusion with a reduced daily dose and gradually increase it to the maximum dose (see previous point).
Form and Duration of Administration
For single use.
Once the bag is opened, it is recommended to use its contents immediately and not store them for subsequent infusions.
The appearance of the mixture after reconstitution is a homogeneous emulsion similar to milk.
For instructions on the preparation and handling of the emulsion for infusion, see section 6.6 of the Technical Sheet.
Due to its high osmolality, Olimel can only be administered through a central vein.
The recommended duration of infusion of a parenteral nutrition bag is between 12 and 24 hours.
Treatment with parenteral nutrition can continue for as long as the patient's clinical condition requires.
- Incompatibilities
No other medicinal product or drug should be added to any of the components of the bag or the reconstituted emulsion without first confirming the compatibility and stability of the resulting preparation (in particular the stability of the lipid emulsion).
Incompatibilities may occur due to, for example, excessive acidity (low pH) or inadequate content of divalent cations (Ca2+ and Mg2+), which can destabilize the lipid emulsion.
As with any parenteral nutrition mixture, the calcium and phosphate ratios should be taken into account. Excessive addition of calcium and phosphate, especially in the form of mineral salts, can cause the formation of calcium phosphate precipitates.
Olimel contains calcium ions, which poses an additional risk of coagulation in citrate-anticoagulated/conserved blood or its components.
Ceftriaxone should not be mixed or administered with intravenous solutions containing calcium, including Olimel, through the same infusion line (e.g., Y-connector) due to the risk of ceftriaxone precipitation with calcium salt (see sections 4.4 and 4.5 of the Technical Sheet). Ceftriaxone and calcium-containing solutions can be administered sequentially one after the other if different infusion lines are used, or if the infusion lines are replaced or flushed.
Due to the risk of precipitation, Olimel should not be administered through the same infusion line or mixed with ampicillin or fosphenytoin.
Check the compatibility with solutions administered simultaneously through the same administration equipment, catheter, or cannula.
It should not be administered before, simultaneously, or after blood through the same equipment due to the risk of pseudoagglutination.
- Special Precautions for Disposal and Other Handling
In Table 1, an overview of the preparation steps for the administration of Olimel is provided.
To Open
Remove the protective outer bag.
Discard the sachet with the oxygen absorber.
Confirm the integrity of the bag and the non-permanent seals. Use it only if the bag is not damaged, and the non-permanent seals are intact (i.e., they have not been opened).
After mixing the contents of the three compartments), if the amino acid solution and the glucose solution are transparent, colorless or slightly yellowish, practically free of visible particles and if the lipid emulsion is a homogeneous liquid with a milky appearance.
Mixing of the solutions and the emulsion
Make sure the product is at room temperature when breaking the non-permanent seals.
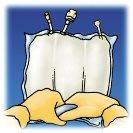
Manually roll the bag over itself, starting from the top of the bag (hanger end). The non-permanent seals will disappear from the side close to the entrances. Continue rolling until the seals open approximately halfway through their length.
Mix the bag by inverting it at least 3 times.
The appearance after reconstitution is a homogeneous emulsion similar to milk.
Additions
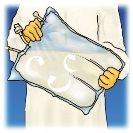
The bag has sufficient capacity for vitamins, electrolytes, and trace elements to be added.
Any addition (including vitamins) must be made to the reconstituted mixture (after opening the non-permanent seals and mixing the contents of the three compartments).
Vitamins can also be added to the glucose compartment before reconstituting the mixture (before opening the non-permanent seals and mixing the contents of the three compartments).
When additions are made to formulations containing electrolytes, the amount of electrolytes already present in the bag must be taken into consideration.
Additions must be carried out by qualified personnel under aseptic conditions.
Olimel can be supplemented with electrolytes according to the following table:
Per 1000 ml | |||
Included level | Maximum additional amount | Maximum total level | |
Sodium | 35 mmol | 115 mmol | 150 mmol |
Potassium | 30 mmol | 120 mmol | 150 mmol |
Magnesium | 4.0 mmol | 1.6 mmol | 5.6 mmol |
Calcium | 3.5 mmol | 1.5 (0.0(a)) mmol | 5.0 (3.5(a)) mmol |
Inorganic phosphate | 0 mmol | 3.0 mmol | 3.0 mmol |
Organic phosphate | 15 mmol(b) | 10 mmol | 25 mmol(b) |
a Value corresponding to the addition of inorganic phosphate
b Including phosphate provided by the lipid emulsion
Trace elements and vitamins:
Stability has been demonstrated with commercially available vitamin and trace element preparations (containing up to 1 mg of iron).
Compatibility with other additives can be consulted upon request.
When making additions, the final osmolarity of the mixture should be measured before administering it through a peripheral vein.
To make an addition:
- It must be carried out under aseptic conditions.
- Prepare the injection point of the bag.
- Puncture the injection point and inject the additives using a syringe or a reconstitution device.
- Mix the contents of the bag and the additives.
Preparation for infusion
It must be carried out under aseptic conditions.
Hang the bag.
Remove the plastic protector from the administration outlet.
Firmly insert the tip of the infusion equipment into the administration outlet.
Table 1: Preparation steps for the administration of Olimel
1. |
| 2. |
| 3. |
|
Break from the top to open the overbag. | Remove the front of the overbag to access the Olimel bag. Discard the overbag and the oxygen packet. | Place the bag on a horizontal and clean surface with the handle facing you. | |||
4. |
| 5. |
| 6. |
|
Lift the hanger area to remove the solution from the top of the bag. Roll the top of the bag firmly until the seals are completely open (approximately halfway). | Mix the contents by inverting the bag at least 3 times. | Hang the bag. Turn the protector to remove it from the administration outlet. Firmly connect the spike connector. |
Administration
For single use only.
Administer the product only after the non-permanent seals between the three compartments have been broken and the contents of the three compartments have been mixed.
Ensure that the final emulsion for infusion does not show any phase separation.
After opening the bag, the contents must be used immediately. The opened bag should never be stored for later infusion. Do not reconnect a partially used bag.
Do not connect bags in series to avoid gas embolism due to the gas present in the first bag.
All unused medication, materials that have come into contact with it, and all necessary devices must be discarded.
Extravasation
The catheter area should be regularly inspected to identify signs of extravasation.
If extravasation occurs, administration should be stopped immediately, keeping the cannula or catheter in place for immediate patient treatment. If possible, aspiration should be performed through the inserted cannula/catheter to reduce the amount of fluid present in the tissues before removing the cannula/catheter.
Specific measures should be taken depending on the stage or extent of any injury caused by the extravasated product (including the products mixed with Olimel).
Treatment options may include pharmacological, non-pharmacological methods, and/or surgical intervention. In the case of significant extravasation, a plastic surgeon should be consulted within the first 72 hours.
The extravasation area should be inspected at least every 4 hours during the first 24 hours and then once a day.
Infusion should not be resumed in the same central vein.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OLIMEL N9E EMULSION FOR PERFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for OLIMEL N9E EMULSION FOR PERFUSION
Discuss questions about OLIMEL N9E EMULSION FOR PERFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions