
OLFEN 140 MG APOSITOS ADHESIVOS MEDICAMENTOSOS

Cómo usar OLFEN 140 MG APOSITOS ADHESIVOS MEDICAMENTOSOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Olfen 140 mg apósitos adhesivos medicamentosos
diclofenaco sódico
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 3 días.
Contenido del prospecto:
- Qué es Olfen y para qué se utiliza
- Qué necesita saber antes de empezar a usar Olfen
- Cómo usar Olfen
- Posibles efectos adversos
- Conservación de Olfen
- Contenido del envase e información adicional
1. Qué es Olfen y para qué se utiliza
Olfen es un medicamento que reduce el dolor. Pertenece al grupo de los antiinflamatorios no esteroideos (AINES).
Para adultos y adolescentes a partir de 16 años de edad:
Este medicamento se utiliza para el tratamiento a corto plazo en el tratamiento sintomático local del dolor agudo asociado a torceduras, esguinces o magulladuras en brazos y piernas como resultado de lesiones, por ejemplo, lesiones deportivas.
2. Qué necesita saber antes de empezar a usar Olfen
No use Olfen
- si es alérgico a diclofenaco, propilenglicol, butilhidroxitolueno o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6),
- si es alérgico a cualquier otro antiinflamatorio no esteroideo (AINE, por ejemplo, ácido acetilsalicílico, ibuprofeno)
- si desarrolló asma, erupción de la piel o inflamación e irritación dentro de la nariz después de tomar ácido acetilsalicílico o cualquier otro AINE,
- si padece úlcera péptica activa,
- si tiene la piel con alguna herida abierta (por ejemplo, abrasiones en la piel, cortes, quemaduras), infecciones en la piel o eccema,
- durante los tres últimos meses de embarazo.
- en niños y adolescentes menores de 16 años
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento
- si padece o ha padecido previamente asma bronquial o alergias, puede experimentar espasmos del músculo bronquial (broncoespasmo), que se manifiesta con dificultades para respirar,
- si observa erupción cutánea retire inmediatamente el apósito adhesivo medicamentoso y suspenda el tratamiento,
- si padece trastornos de los riñones, corazón o hígado o si padece o ha padecido previamente de úlcera gastrointestinal, inflamación intestinal o tendencia al sangrado.
Los efectos adversos pueden reducirse empleando la mínima dosis eficaz durante el menor período de tiempo posible.
Precauciones IMPORTANTES:
- Si los síntomas persisten durante más de 3 días o empeoran, debe consultar con su médico,
No use el parche adhesivo medicamentoso en los ojos o membranas mucosas o permita que entre en contacto con ellos
Después de retirar el apósito adhesivo medicamentoso evite la exposición de la zona tratada directamente a la luz solar o a otras fuentes de rayos ultravioletas (por ejemplo solarium) durante al menos un día para reducir el riesgo de sensibilidad a la luz.
No usar de forma simultánea, ni vía tópica o vía sistémica, ningún medicamento conteniendo diclofenaco u otros AINES
Pacientes de edad avanzada
Los pacientes de edad avanzada deben usar este medicamentocon precaución, ya que son más propensos a experimentar efectos adversos.
Niños y adolescentes
Este medicamento no debe ser usado en niños y adolescentes menores de 16 años debido a la falta de experiencia.
Uso de Olfen con otros medicamentos
Informe a su médico o farmacéutico si está utilizando/tomando, ha utilizado recientemente o pudiera tener que utilizar/tomar cualquier otro medicamento.
Siempre que el medicamento se use correctamente, la absorción de diclofenaco por el cuerpo es muy pequeña, por lo que las interacciones descritas cuando el diclofenaco se administra de forma oral es poco probable que ocurran.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
Durante los seis primeros meses de embarazo, sólo tras consultar con su médico puede usar Olfen apósitos adhesivos medicamentosos.
Durante el último trimestre de embarazo, no debe usar Olfen, ya que existe un incremento de riesgo de complicaciones para la madre y el niño (ver “No use Olfen”).
Lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Cantidades muy pequeñas de diclofenaco se excretan en la leche materna. Como no se conocen efectos adversos en los niños, en general no es necesario interrumpir la lactancia durante el uso de Olfen. Sin embargo, el apósito no debe aplicarse directamente en la zona de la mama.
Conducción y uso de máquinas:
Este medicamento tiene una influencia nula o insignificante en su capacidad para conducir y utilizar máquinas.
Olfen contiene propilenglicol y butilhidroxitolueno,
Este medicamento contiene 1.400 mg de propilenglicol en cada adhesivo. El propilenglicol puede producir irritación de la piel..
El butilhidroxitolueno puede producir reacciones locales en la piel (como dermatitis por contacto) o irritación de los ojos y mucosas.
3. Cómo usar Olfen
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
La dosis recomendada es:
Aplicar un apósito adhesivo medicamentoso en la zona dolorida dos veces al día, por la mañana y por la noche. La dosis máxima diaria total es de 2 apósitos medicamentosos, incluso si hay más de un área lesionada para ser tratada. Tratar sólo un área dolorida cada vez.
Uso en niños y adolescentes
No existen datos suficientes sobre la eficacia y seguridad en niños ni en adolescentes menores de 16 años.
Método de administración
Sólo para uso cutáneo. ¡No ingerir!
- Cortar el sobre que contiene el apósito adhesivo medicamentoso a lo largo de la marca.
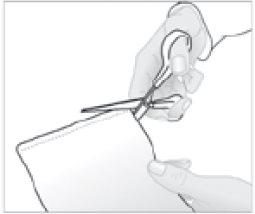
- Saque el apósito adhesivo medicamentoso y cierre cuidadosamente el sobre presionado en el cierre

- Retire la película protectora de la superficie del apósito adhesivo medicamentoso.
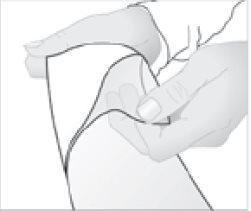
- Coloque el apósito adhesivo medicamentoso sobre la zona dolorida.
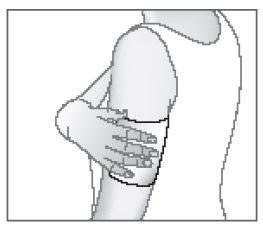
Si fuera necesario, el apósito adhesivo medicamentoso puede ser adherido utilizando un vendaje elástico en forma de malla.
No utilice el apósito adhesivo medicamentoso junto a un vendaje oclusivo.
No debe dividir el apósito adhesivo medicamentoso.
El apósito adhesivo medicamentoso usado debe ser doblado por la mitad, con el lado adhesivo hacia el interior.
Duración de uso
No use Olfen durante más de 3 días sin el consejo de su médico. El uso de este medicamento durante más tiempo debe ser consultado con el médico y no debe exceder de 7 días.
Si usa más Olfen del que debiera
Si ha usado más Olfen del que debiera, consulte inmediatamente a su médico o a su farmacéutico o al Servicio de Información Toxicológica (Tel: 91 562 04 20).
Informe a su médico si ocurren efectos adversos graves tras el uso incorrecto del medicamento o tras sobredosis accidental (por ejemplo en niños). Su médico le aconsejará de las medidas que sean necesarias, dependiendo de la gravedad de la intoxicación.
Si olvidó usar Olfen
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamentopuede producir efectos adversos, aunque no todas las personas los sufran.
Consulte con su médico inmediatamente y suspenda el uso del apósito adhesivo medicamentoso, si nota cualquiera de los siguientes:
Repentina erupción cutánea con picor (urticaria), hinchazón de las manos, pies, tobillos, cara, labios, boca o garganta; dificultad para respirar, caída de la presión arterial o debilidad.
Puede experimentar los siguientes efectos adversos:
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
Reacciones locales de la piel, como enrojecimiento de la piel, sensación de quemazón, picor, enrojecimiento de la piel inflamada, erupción cutáneas, a veces con pústulas o pápulas.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
Reacciones de hipersensibilidad o reacciones alérgicas locales (dermatitis de contacto).
Frecuencia no conocida (no se puede estimar a partir de los datos disponibles)
Piel seca
En pacientes que han utilizado tópicamente sustancias activas pertenecientes al mismo grupo de medicamentos que diclofenaco, se han producido casos aislados de erupción cutánea generalizada, reacciones de hipersensibilidad, como hinchazón de la piel y membranas mucosas y reacciones de tipo anafiláctico con trastornos agudos circulatorios y sensibilidad a la luz.
La absorción de diclofenaco en el cuerpo a través de la piel es muy baja en comparación con la concentración de la sustancia activa en la sangre tras la ingesta oral de diclofenaco. La probabilidad de efectos adversos en el cuerpo (como alteraciones renales o gastrointestinales o dificultades para respirar) es muy baja.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad del medicamento.
5. Conservación de Olfen
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de la abreviatura CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC.
Conservar en el embalaje original para protegerlo de la luz y de la desecación.
Guardar el sobre herméticamente cerrado para protegerlo de la desecación.
Puede almacenarse durante 4 meses después de la primera apertura del sobre.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Olfen
El principio activo es: diclofenaco sódico. Cada apósito adhesivo medicamentoso contiene 140 mg de diclofenaco sódico.
Los demás componentes son: glicerol, propilenglicol (E1520), diisopropil adipato, sorbitol líquido cristalizable (E-420), carmelosa sódica, poliacrilato sódico, copolímero base de metacrilato de butilo, edetado de disodio, sulfito de sodio (E-221), butilhidroxitolueno (E-321), sulfato seco de aluminio potásico, sílice coloidal anhidra, caolín ligero (natural), macrogol éter laurílico (9 unidades EO), levomentol, ácido tartárico, agua purificada, soporte no tejido de poliester, lámina protectora de polipropileno.
Aspecto del producto y contenido del envase:
Olfen son apósitos de 10 x 14 cm con una pasta de color blanco a marrón claro uniformemente extendida como una base uniforme en un soporte no tejido y con una película protectora desechable.
Olfen se encuentra disponible en envases con 2, 5, 10 ó 14 apósitos adhesivos en sobres de autocierre conteniendo 2 ó 5 apósitos medicamentosos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular
Teva Pharma, S.L.U.
C/ Anabel Segura 11, Edificio
Albatros B 1ª planta 28108
Alcobendas, Madrid (España)
Responsable de la fabricación
Merckle GmbH
Ludwing-Merckle-Strabe 3
89143 Blaubeuren, (Alemania)
Este medicamento está autorizado en los Estados Miembros del EEE con los siguientesnombres:
Austria: Dolostrip 140 mg wirkstoffhaltiges Pflaster
Bélgica: Kinespir Patch 140 mg pleister
República Checa: Olfen 140 mg lécivé náplasti
Dinamarca: Diclofenac ratiopharm
Alemania: Diclofenac-ratiopharm Schmerzpflaster
Hungría: Algoplast-ratiopharm 140 mg gyógyszeres tapasz
Italia: Diclofenac Pharmentis 140mg cerotti medicati
Eslovaquia: Diclobene 140 mg
España: Olfen 140 mg apósitos adhesivos medicamentosos
Reino Unido: ALGOPAIN-Eze 140 mg medicated plaster
Fecha de la última revisión de este prospecto:Octubre 2019
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://cima.aemps.es/cima/dochtml/p/70307/P_70307.html
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OLFEN 140 MG APOSITOS ADHESIVOS MEDICAMENTOSOSForma farmacéutica: GEL, 10 mg/gPrincipio activo: DiclofenacoFabricante: Laboratorios Cinfa S.A.No requiere recetaForma farmacéutica: GEL, 23,2 mg/gPrincipio activo: DiclofenacoFabricante: Laboratorios Cinfa S.A.No requiere recetaForma farmacéutica: LIQUIDO USO TOPICO, 39,2 mg/mlPrincipio activo: DiclofenacoFabricante: Laboratorios Cinfa S.A.No requiere receta
Médicos online para OLFEN 140 MG APOSITOS ADHESIVOS MEDICAMENTOSOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OLFEN 140 MG APOSITOS ADHESIVOS MEDICAMENTOSOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






