ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ

Запитайте лікаря про рецепт на ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ

Інструкція із застосування ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Введення
ОСНОВНІ ВІДОМОСТІ: ІНФОРМАЦІЯ ДЛЯ КОРИСТУВАЧА
OCTAPLEX500 ОД, порошок і розчинник для інфузійного розчину. Людський протромбіновий комплекс.
OCTAPLEX1000 ОД, порошок і розчинник для інфузійного розчину. Людський протромбіновий комплекс.
Прочитайте уважно весь листок перед тим, як почати використовувати цей лікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей листок, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
- Цей лікарський засіб призначений тільки для вас, і не слід давати його іншим людям, навіть якщо вони мають такі самі симптоми, як і ви, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому листку. Див. розділ 4.
Зміст листка:
- Що таке Octaplex і для чого він використовується
- Що потрібно знати перед тим, як почати використовувати Octaplex
- Як використовувати Octaplex
- Можливі побічні ефекти
- Зберігання Octaplex
- Зміст упаковки та додаткова інформація
1. Що таке Octaplex і для чого він використовується
Octaplex належить до групи лікарських засобів, званих коагуляційними факторами. Він містить людські коагуляційні фактори II, VII, IX і X, залежні від вітаміну К.
Octaplex використовується для лікування та профілактики кровотеч:
- Спровокованих лікарськими засобами, званими антагоністами вітаміну К (наприклад, варфарином). Ці лікарські засоби блокують дію вітаміну К і спричиняють дефіцит коагуляційних факторів, залежних від вітаміну К, в вашому організмі.
Octaplex використовується, коли потрібно швидко виправити дефіцит.
- У осіб, які народилися з цим дефіцитом коагуляційних факторів II і X, залежних від вітаміну К. Він використовується, коли не доступний продукт конкретного коагуляційного фактору, очищений.
2. Що потрібно знати перед тим, як почати використовувати Octaplex
Не використовуйте Octaplex:
- Якщо ви алергічні на людський протромбіновий комплекс або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6).
- Якщо ви алергічні на гепарин або якщо гепарин раніше спричиняв у вас зниження рівня тромбоцитів у крові.
- Якщо у вас є дефіцит IgA з відомими антитілами проти IgA.
Попередження та обережність:
- Проконсультуйтеся з лікарем-спеціалістом з досвідом лікування порушень згортання крові, коли ви прийматимете Octaplex.
- Якщо у вас є набутий дефіцит коагуляційних факторів, залежних від вітаміну К (наприклад, спричинений лікуванням лікарськими засобами-антагоністами вітаміну К), Octaplex можна використовувати тільки у разі необхідності швидкого виправлення дефіциту, наприклад, при важких кровотечах або у разі термінової операції. В інших випадках зазвичай достатньо зменшити дозу антагоністів вітаміну К і/або призначити вітамін К.
- Якщо ви приймаєте лікарські засоби-антагоністи вітаміну К (наприклад, варфарин), у вас може бути підвищений ризик утворення кров'яних згустків. У цьому випадку лікування Octaplex може збільшити ризик.
- Якщо ви народилися з дефіцитом коагуляційних факторів, залежних від вітаміну К, повинен бути використаний конкретний коагуляційний фактор, якщо він доступний.
- Якщо відбувається будь-яка алергічна реакція або анафілактична реакція, інфузію лікарського засобу негайно зупинять і проводять відповідне лікування.
- Існує ризик утворення тромбозу або дисемінованої внутрішньосудинної коагуляції (важкої хвороби, при якій утворюються згустки по всьому тілу) при прийомі Octaplex (особливо якщо ви приймали його регулярно). Вас буде ретельно спостерігати на предмет ознак або симптомів коагуляції або тромбозу.
Це особливо важливо, якщо у вас є історія хвороби коронарних артерій, захворювання печінки, якщо ви підете на операцію, а також якщо Octaplex призначений новонародженим дітям.
- Немає даних про використання Octaplex при кровотечах під час народження через дефіцит вітаміну К у новонароджених.
Вірусна безпека
- Коли лікарські засоби, отримані з плазми або людської крові, вводяться пацієнтам, необхідно проводити певні заходи для запобігання передачі інфекцій пацієнтам. Такі заходи включають ретельний відбір донорів крові та плазми, який гарантує виключення тих, хто має ризик бути носієм інфекцій, та аналіз ознаків вірусів/інфекцій у окремих донорських матеріалах та у сумішах плазми. Виробники цих продуктів також включають етапи обробки крові або плазми, які можуть інактивувати або видалити віруси. Незважаючи на ці заходи, при введенні лікарських засобів, приготовлених з людської крові або плазми, не можна повністю виключити можливість передачі інфекції. Це також стосується всіх невідомих або нових вірусів чи інших типів інфекцій.
Заходи, які приймаються, вважаються ефективними щодо вірусів з оболонкою, таких як вірус імунодефіциту людини (ВІЛ), вірус гепатиту Б (ВГБ) та вірус гепатиту С (ВГС). Заходи, які приймаються, можуть мати обмежену цінність щодо вірусів без оболонки, таких як вірус гепатиту А (ВГА) та парвовірус В19. Інфекція парвовірусом В19 може бути серйозною для вагітної жінки (фетальна інфекція) та для осіб з імунодефіцитом або тих, хто страждає на деякі види анемій (наприклад, хвороба дреपनоціту або гемолітична анемія).
Рекомендується записувати назву та номер партії продукту щоразу, коли вводиться доза Octaplex, щоб підтримувати зв'язок з використаними партіями.
- Рекомендується проводити відповідну вакцинацію (гепатит А та Б) якщо вам регулярно або повторно вводять людський протромбіновий комплекс.
Діти та підлітки
Немає даних про використання Octaplex у дітей та підлітків.
Інші лікарські засоби та Octaplex
- Octaplex не слід змішувати з іншими лікарськими засобами.
- Octaplex блокує дію лікарських засобів-антагоністів вітаміну К (наприклад, Варфарину), але не відомо про взаємодію з іншими лікарськими засобами.
- Octaplex може впливати на результати тестів згортання крові, які чутливі до гепарину.
- Повідомте вашому лікареві або фармацевту, якщо ви приймаєте або нещодавно приймали інші лікарські засоби чи якщо вам потрібно приймати інші лікарські засоби.
Вагітність та лактація
- Octaplex слід використовувати під час вагітності та лактації тільки у разі явної необхідності. Проконсультуйтеся з вашим лікарем або фармацевтом перед використанням будь-якого лікарського засобу.
Водіння транспортних засобів та використання машин:
Не описано, що Octaplex впливає на здатність водіння транспортних засобів та використання машин.
Важлива інформація про деякі компоненти Octaplex
Гепарин може спричиняти алергічні реакції та зниження кількості тромбоцитів у крові, що може вплинути на систему згортання крові. Пацієнти з історією алергічних реакцій, індукованих гепарином, повинні уникати використання лікарських засобів, які містять гепарин.
Цей лікарський засіб містить 75–125 мг (флакон 500 ОД) або 150–250 мг (флакон 1000 ОД) натрію (основний компонент кухонної солі) на флакон. Це відповідає 3,8–6,3% або 7,5–12,5% від максимальної добової норми споживання натрію, рекомендованої для дорослих.
3. Як використовувати Octaplex
Лікування Octaplex повинно проводитися під наглядом лікаря з досвідом лікування порушень згортання крові.
- Спочатку порошок розчиняють у воді.
- Потім розчин вводять через вену (в/в).
Кількість Octaplex, яку ви отримуватимете, і тривалість лікування залежатимуть від:
- серйозності вашої хвороби
- місця розташування кровотечі та її тяжкості, а також
- вашого загального стану.
Якщо ви використовуєте більше Octaplex, ніж потрібно
У разі передозування збільшується ризик розвитку:
- ускладнень згортання крові (наприклад, інфаркту міокарда та утворення згустків у ваших венах або легенях)
- дисемінованої внутрішньосудинної коагуляції (важкої хвороби, при якій утворюються згустки по всьому тілу).
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може спричиняти побічні ефекти, хоча не всі люди їх відчувають.
Часті: (можуть впливати до 1 особи з 10)
Згустки у кровоносних судинах
Рідкі: (можуть впливати до 1 особи з 100)
Тревога, підвищення артеріального тиску, симптоми, подібні до астми, кашель з кров'ю, носова кровотеча, печія в місці ін'єкції та згустки в апараті.
Рідкісні (можуть впливати до 1 особи з 1000)
Можуть виникнути алергічні реакції.
Рідко спостерігається тимчасове підвищення результатів тестів на функцію печінки (трансаміназ).
Пацієнти, які приймають Octaplex для заміни терапії, можуть розвивати нейтралізуючі антитіла (інгібітори) проти одного або декількох коагуляційних факторів, які містяться в препараті. Якщо ці інгібітори з'являються, заміна терапії не буде дуже ефективною.
Дуже рідкісні (можуть впливати до 1 особи з 10 000)
Спостерігається підвищення температури тіла (лихоманка).
Існує ризик згортання крові після введення цього лікарського засобу.
Частота невідома: (не може бути оцінена на підставі доступних даних).
Важка алергічна реакція та шок, гіперчутливість, тремор, серцева недостатність, підвищення частоти серцевих скорочень, порушення кровообігу, зниження артеріального тиску, порушення дихання, труднощі з диханням, нудота, блювання, висипання на шкірі, озноб.
Гепарин у цьому препараті може спричиняти раптове зниження кількості тромбоцитів у крові. Це алергічна реакція, яку називають «тромбоцитопенія, індукована гепарином типу II». Рідко у пацієнтів, які раніше не мали гіперчутливості до гепарину, це зниження кількості тромбоцитів може виникнути між 6-м і 14-м днем після початку лікування. У пацієнтів з попередньою гіперчутливістю до гепарину ця зміна може розвинутися протягом годин після початку лікування.
Лікування Octaplex повинно бути негайно зупинено у пацієнтів, які демонструють цю алергічну реакцію. Цим пацієнтам не слід приймати лікарські засоби, які містять гепарин, у майбутньому.
Для інформації про вірусну безпеку див. розділ 2.
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому листку. Ви також можете повідомити про них безпосередньо через www.notificaRAM.es. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання Octaplex
Тримайте цей лікарський засіб поза досяжністю дітей.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, вказаного на упаковці. Термін придатності – останній день місяця, вказаного на упаковці.
Тримайте флакон у зовнішній упаковці, щоб захистити його від світла, та при температурі нижче 25°C. Не заморожуйте.
Порошок повинен бути розчинений завжди безпосередньо перед ін'єкцією. Стабільність розчину доведена протягом максимум 8 годин при 25°C. Однак, щоб запобігти забрудненню, розчин повинен бути використаний негайно та лише один раз.
6. Зміст упаковки та додаткова інформація
Склад Octaplex на флакон і після реконституції з 20 мл (500 ОД)/40 мл (1000 ОД) розчинника:
Активними речовинами є:
Назва компонента | Octaplex Кількість на флакон 500 ОД | Octaplex Кількість на флакон 1000 ОД | OCTAPLEX Кількість на мл реконституйованого розчину |
Загальні білки: | 260–820 мг | 520–1640 мг | 13–41 мг/мл |
Активні речовини | |||
Людський коагуляційний фактор II | 280–760 ОД | 560–1520 ОД | 14–38 ОД/мл |
Людський коагуляційний фактор VII | 180–480 ОД | 360–960 ОД | 9–24 ОД/мл |
Людський коагуляційний фактор IX | 500 ОД | 1000 ОД | 25 ОД/мл |
Людський коагуляційний фактор X | 360–600 ОД | 720–1200 ОД | 18–30 ОД/мл |
Інші активні компоненти | |||
Білок С | 260–620 ОД | 520–1240 ОД | 13–31 ОД/мл |
Білок С | 240–640 ОД | 480–1280 ОД | 12–32 ОД/мл |
Специфічна активність фактору IX ? 0,6 ОД/мг білка.
Іншими компонентами є гепарин, трисодійний цитрат дигідрат і вода для ін'єкцій.
Вигляд продукту та вміст упаковки
Octaplex представлений у вигляді порошку та розчинника для інфузійного розчину. Це гігроскопічний білий або легкий порошок або крихка маса у скляному флаконі. Розчинник – вода для ін'єкцій, яку поставляють у скляному флаконі. Реконституйований розчин прозорий або легкий опалесцентний і може мати колір.
Octaplex поставляється в упаковці, яка містить:
- 1 флакон з порошком для ін'єкційного розчину.
- 1 флакон з розчинником, водою для ін'єкцій
- 1 трансферний набір Nextaro®.
Уповноважений представник:
Octapharma S.A.
Avda. Castilla, 2. (P.E. San Fernando)
Ed. Dublin – 2ª Planta
28830 San Fernando de Henares
Мадрид
Виробник:
Octapharma Pharmazeutika Produktionsges.m.b.H.Oberlaaer Str. 2351100 ViennaАвстрія
або
Octapharma Lingolsheim S.A.S.
72 Rue du Maréchal Foch
67380 Lingolsheim
Франція
Цей лікарський засіб дозволений у країнах-членах Європейського економічного простору під наступними назвами:
Австрія, Бельгія, Болгарія, Кіпр, Хорватія, Данія, Естонія, Фінляндія, Франція, Німеччина, Угорщина, Ісландія, Ірландія, Латвія, Литва, Люксембург, Мальта, Нідерланди, Норвегія, Польща, Португалія, Республіка Словенія, Республіка Словаччина, Іспанія, Велика Британія: Octaplex
Чехія, Швеція: Ocplex
Італія та Румунія: Pronativ
Дата останнього перегляду цього листка: 06/2024
ІНСТРУКЦІЇ ДЛЯ МЕДИЧНИХ ПРАЦІВНИКІВ
Загальна інформація про використання Octaplex наводиться в розділі 3.
Наступна інформація призначена лише для медичного працівника:
Інструкції для лікування
Прочитайте всі інструкції та дотримуйтесь їх ретельно.
Під час процедури, описаної нижче, слід дотримуватися асептичної техніки.
Продукт реконституюється швидко при кімнатній температурі.
Реконституйований розчин повинен бути прозорим або легким опалесцентним. Не використовувати розчини, які є мутними або мають осад. Реконституйовані продукти повинні бути візуально перевірені на наявність частинок та зміни кольору перед введенням.
Після реконституції розчин слід використовувати негайно.
Весь непотрібний продукт або відходи повинні бути видалені згідно з місцевими вимогами.
Дозування:
Кровотеча та профілактика кровотечі під час лікування антагоністами вітаміну К:
Доза залежить від міжнародного нормалізованого відношення (МНВ) до лікування та ваги тіла. У наступній таблиці наводяться приблизні дози (одиниці/кг ваги тіла реконституйованого продукту).
МНВ до лікування | 2 – <4 | 4 – 6 | > 6 |
Доза Octaplex (одиниці* фактору IX)/кг ваги тіла) | 25 | 35 | 50 |
* Одиниці означають міжнародні одиниці (ОД).
Доза базується на вагі тіла до максимальної ваги 100 кг. Таким чином, для пацієнтів, які важать більше 100 кг, максимальна доза (ОД фактору IX) не повинна перевищувати 2500 ОД для МНВ 2 - <4, 3500 ОД для МНВ 4 - 6 та 5000 ОД для МНВ > 6.
Оскільки ці рекомендації є емпіричними, а відновлення та тривалість дії можуть варіюватися, обов'язкове моніторинг МНВ під час лікування.
Кровотеча та профілактика кровотечі під час операції при вроджених дефектах коагуляційних факторів II та X, коли немає доступного конкретного коагуляційного фактору:
Розрахунок необхідної дози для лікування базується на емпіричному даних, що приблизно 1 ОД фактору II або X на кг ваги тіла збільшує плазмову активність фактору II або X на 0,02 та 0,017 ОД/мл, відповідно.
?Одиниці, необхідні = вага тіла (кг) x бажане збільшення фактору X (ОД/мл) x 60
де 60 (мл/кг) – це обернена величина оціненої відновлення.
?Одиниці, необхідні = вага тіла (кг) x бажане збільшення фактору II (ОД/мл) x 50
Якщо відоме індивідуальне відновлення, слід використовувати це значення у розрахунку.
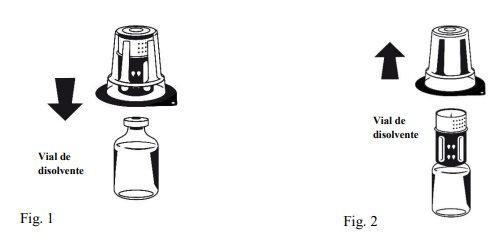
Інструкції щодо реконструкції:
| |
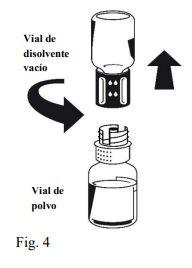
Octaplex швидко розчиняється при кімнатній температурі з утворенням безбарвного або легкого синього розчину. Відкрутіть дві частини Nextaro® (Фіг. 4). Викиньте порожній флакон з розчинником з синьою частиною Nextaro®. | |
Якщо концентрат не розчиняється повністю або утворюється агрегат, не слід використовувати цю підготовку.
Інструкції щодо перфузії:
Як заходи обережності, слід виміряти пульс пацієнтів до та під час перфузії. Якщо відбувається значне збільшення пульсу, слід зменшити швидкість перфузії або перервати введення.
Після того, як розчин було перенесено, міцно утримуйте поршень шприца (тримаючи його вниз) та витягніть шприц з Nextaro®. Викиньте Nextaro® та порожній флакон. |
- Продезінфікуйте відповідним чином зону, де буде застосовуватися ін'єкція.
- Введіть розчин внутрішньовенно зі швидкістю 0,12 мл/кг/хв (≈ 3 одиниці/кг/хв), до максимальної швидкості 8 мл/хв (≈ 210 одиниць/хв), використовуючи асептичну техніку.
Не повинно бути кровотоку до шприца через ризик утворення фібринових згустків. Nextaro® призначений для одноразового використання.
Детальна та оновлена інформація про цей лікарський засіб доступна на сайті Іспанського агентства з лікарських засобів та медичних продуктів (AEMPS)
http://www.aemps.gob.es/
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 1000 МО/флаконДіючі речовини: coagulation factor IX, II, VII and X in combinationВиробник: Csl Behring GmbhПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 500 МОДіючі речовини: coagulation factor IX, II, VII and X in combinationВиробник: Csl Behring GmbhПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 250 МОДіючі речовини: coagulation factor IX, II, VII and X in combinationВиробник: Prothya Biosolutions Netherlands B.V.Потрібен рецепт
Аналоги ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Польща
Аналог ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Україна
Лікарі онлайн щодо ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ОКТАПЛЕКС, 500 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ – за рішенням лікаря та згідно з місцевими правилами.