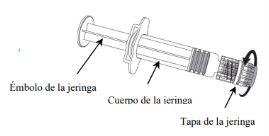
NIMENRIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE EN JERINGA PRECARGADA


Cómo usar NIMENRIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el usuario
Nimenrix polvo y disolventepara solución inyectable en jeringa precargada
Vacuna conjugada frente a meningococo de los grupos A, C, W-135 e Y
Lea todo el prospecto detenidamente antes de que reciba esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted o su hijo y no debe dárselo a otras personas.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Este prospecto se ha escrito asumiendo que la persona que va a recibir la vacuna es la que lo va a leer. No obstante, la vacuna se puede administrar a adultos y niños, de modo que es posible que usted lo lea por su hijo.
Contenido del prospecto
- Qué es Nimenrix y para qué se utiliza
- Qué necesita saber antes de recibir Nimenrix
- Cómo se administra Nimenrix
- Posibles efectos adversos
- Conservación de Nimenrix
- Contenido del envase e información adicional
1. Qué es Nimenrix y para qué se utiliza
Qué es Nimenrix y para que se utiliza
Nimenrix es una vacuna que ayuda a proteger frente a las infecciones causadas por la bacteria (germen) llamada “Neisseria meningitidis”de los tipos A, C, W-135 e Y.
“Neisseria meningitidis”de los tipos A, C, W-135 e Y puede producir enfermedades graves tales como:
- meningitis – una infección del tejido que recubre el cerebro y la médula espinal.
- septicemia – una infección de la sangre.
Estas infecciones se transmiten fácilmente de una persona a otra y, si no se tratan, pueden ocasionar la muerte.
Nimenrix puede administrarse a adultos, adolescentes, niños y lactantes a partir de las 6 semanas de edad.
Cómo funciona Nimenrix
Nimenrix ayuda a su organismo a producir su propia protección (anticuerpos) frente a las bacterias. Estos anticuerpos le ayudan a protegerse frente a las enfermedades.
Nimenrix únicamente le protegerá frente a las infecciones causadas por la bacteria “Neisseria meningitidis”de los tipos A, C, W-135 e Y.
2. Qué necesita saber antes de recibir Nimenrix
No deben administrarle Nimenrix si:
- es alérgico a los principios activos o a alguno de los demás componentes de esta vacuna (incluidos en la sección 6).
Los signos de una reacción alérgica pueden incluir erupción cutánea con picor, dificultad para respirar e hinchazón de la cara o de la lengua. Acuda a su médico inmediatamente si experimenta cualquiera de estos signos.
Si no está seguro, hable con su médico o enfermero antes de que le administren Nimenrix.
Advertencias y precauciones
Consulte a su médico o enfermero antes de que le administren esta vacuna si:
- tiene una infección con fiebre elevada (de más de 38 °C). Si este es su caso, no deben administrarle la vacuna hasta que se encuentre mejor. Una infección de poca importancia, como un resfriado, no debería ser un problema. No obstante, consulte antes con su médico o enfermero.
- tiene un problema de coagulación o le aparecen cardenales con facilidad.
Si se encuentra en cualquiera de las circunstancias anteriores (o no está seguro), consulte con su médico o enfermero antes de que le administren Nimenrix.
Puede que Nimenrix no proteja por completo a todos los vacunados. Si usted tiene un sistema inmune débil (por ejemplo, debido a una infección por VIH o a medicamentos que afectan al sistema inmune) es posible que no se beneficie al máximo de la vacunación con Nimenrix.
Antes o después de cualquier inyección, podría producirse un desmayo (especialmente en los adolescentes), por lo que debe informar a su médico o enfermero si usted o su hijo se ha desmayado en anteriores ocasiones tras la administración de una inyección.
Otros medicamentos yNimenrix
Informe a su médico o enfermero si está utilizando o ha utilizado recientemente cualquier otro medicamento, incluyendo otras vacunas y medicamentos adquiridos sin receta.
Puede que Nimenrix no sea tan eficaz si está utilizando medicamentos que afecten a su sistema inmune.
En lactantes, Nimenrix se puede administrar simultáneamente con vacunas combinadas difteria – tétanos – tosferina acelular (DTPa), incluyendo tosferina acelular (DTPa) con hepatitis B, poliovirus inactivado o Haemophilus influenzaetipo b (VHB, IPV o Hib), como la vacuna DTPa-VHB-IPV/Hib y la vacuna conjugada antineumocócica 10-valente.
Desde 1 año de edad y mayores, Nimenrix se puede administrar al mismo tiempo que con alguna de las siguientes vacunas: hepatitis A (VHA) y hepatitis B (VHB), la vacuna del sarampión-paperas-rubeola (SRP, triple vírica), la vacuna del sarampión-paperas-rubeola-varicela (SRPV), la vacuna conjugada antineumocócica 10-valente o la vacuna antigripal estacional no adyuvada.
En el segundo año de vida, Nimenrix también se puede administrar al mismo tiempo con difteria – tétanos – tosferina acelular (DTPa), incluyendo tosferina acelular (DTPa) con hepatitis B, poliovirus inactivado o Haemophilus influenzaetipo b (VHB, IPV o Hib), como la vacuna DTPa-VHB-IPV/Hib y la vacuna conjugada antineumocócica 13-valente.
En personas de entre 9 y 25 años, Nimenrix se puede administrar al mismo tiempo que la vacuna del virus del papiloma humano [tipos 16 y 18] y una vacuna combinada de difteria (contenido de antígeno reducido), tétanos y tosferina acelular.
Siempre que sea posible, la administración de Nimenrix y una vacuna que contenga toxoide tetánico, como la vacuna DTPa-VHB-IPV/Hib, se realizará al mismo tiempo o Nimenrix se administrará al menos un mes antes que la vacuna que contenga toxoide tetánico.
Cada vacuna se administrará en lugares de inyección diferentes.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada, tiene intención de quedarse embarazada, o está en periodo de lactancia, consulte a su médico antes de recibir Nimenrix.
Conducción y uso de máquinas
No es probable que Nimenrix afecte a su capacidad para conducir o usar máquinas. No obstante, no conduzca o use máquinas si no se encuentra bien.
Nimenrix contiene sodio
Este medicamento contiene menos de 23 mg (1 mmol) de sodio por dosis: esto es, esencialmente “exento de sodio”.
3. Cómo se administra Nimenrix
Su médico o enfermero le administrará Nimenrix.
Nimenrix siempre se inyecta en un músculo, normalmente en la parte superior del brazo o muslo.
Primovacunación
Lactantes de 6 semanas a menos de 6 meses de edad
Dos inyecciones administradas con 2 meses de diferencia, por ejemplo a los 2 y 4 meses de edad (la primera inyección se puede administrar a partir de las 6 semanas de edad).
Lactantes de 6 meses de edad, niños, adolescentes y mayores
Una inyección.
Dosis de refuerzo
Lactantes de 6 semanas a menos de 12 meses de edad:
Una dosis de refuerzo a los 12 meses de edad, al menos 2 meses después de la última dosis de Nimenrix.
Personas previamente vacunadas de 12 meses de edad y mayores:
Informe a su médico si le han administrado anteriormente una inyección de otra vacuna antimeningocócica distinta de Nimenrix.
Su médico le indicará si necesita una inyección adicional de Nimenrix y cuándo la necesita, especialmente si usted o su hijo:
- recibió la primera dosis a los 6-14 meses de edad y podría tener un riesgo aumentado de infección causada por Neisseria meningitidisde los tipos W-135 o Y
- recibió la dosis hace más de un año aproximadamente y podría tener riesgo de infección causada por Neisseria meningitidisdel tipo A
- recibió la primera dosis a los 12-23 meses de edad y podría tener un riesgo aumentado de infección causada por Neisseria meningitidisde los tipos A, C, W-135 o Y
Se le informará cuando debe regresar usted o su hijo para que le administren su próxima inyección. Si usted o su hijo no recibe una de las inyecciones programadas, es importante que solicite otra cita.
Asegúrese de que usted o su hijo termina el ciclo completo de vacunación.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Con este medicamento pueden ocurrir los siguientes efectos adversos:
Muy frecuentes (pueden ocurrir con más de 1 de cada 10 dosis de la vacuna):
- fiebre
- cansancio (fatiga)
- dolor de cabeza
- sensación de adormecimiento
- pérdida de apetito
- sensación de irritabilidad
- hinchazón, dolor y enrojecimiento en el lugar en el que se administró la inyección.
Frecuentes (pueden ocurrir hasta con 1 de cada 10 dosis de la vacuna):
- cardenales (hematomas) en el lugar en el que se administró la inyección
- problemas de estómago y de digestión, tales como diarrea, vómitos y náuseas.
- erupción (lactantes)
Poco frecuentes (pueden ocurrir hasta con 1 de cada 100 dosis de la vacuna):
- erupción
- habones
- picor
- llanto no habitual
- sensación de mareo
- músculos doloridos
- dolor en los brazos o en las piernas
- malestar general
- dificultad para dormir
- sensibilidad disminuida, especialmente en la piel
- reacciones en el lugar en el que se administró la inyección, tales como picor, sensación de calor o entumecimiento o aparición de un bulto duro
- reacción alérgica
Raros (pueden ocurrir hasta con 1 de cada 1.000 dosis de la vacuna):
- ataques (convulsiones) relacionados con una fiebre elevada
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- hinchazón en el lugar de la inyección y enrojecimiento; esto puede afectar a un área extensa de la extremidad donde se administra la vacuna
- ganglio linfático inflamado
- reacción alérgica grave
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nimenrix
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 °C y 8 °C).
- Conservar en el embalaje original para protegerla de la luz.
- No congelar.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nimenrix
Tras la reconstitución, 1 dosis (0,5 ml) contiene: Polisacárido de Neisseria meningitidisdel grupo A1 Polisacárido de Neisseria meningitidisdel grupo C1 Polisacárido de Neisseria meningitidisdel grupo Y1 1conjugado con toxoide tetánico como proteína transportadora | 5 microgramos 5 microgramos 5 microgramos 5 microgramos 44 microgramos |
- Los demás componentes son:
- En el polvo: sacarosa y trometamol
- En el disolvente: cloruro de sodio (ver sección 2 “Nimenrix contiene sodio”) y agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Nimenrix es un polvo y disolvente para solución inyectable.
Nimenrix se suministra como un polvo o pasta de color blanco en un vial de vidrio de una sola dosis y un disolvente transparente e incoloro en una jeringa precargada.
Ambos deben mezclarse antes de su uso. La apariencia de la vacuna mezclada será una solución transparente e incolora.
Nimenrix está disponible en envases de 1 o 10 con o sin agujas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización: Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Bruxelles Bélgica | Fabricante responsable de la liberación de los lotes: Pfizer Manufacturing Belgium N.V. Rijksweg 12 2870 Puurs-Sint-Amands Bélgica |
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer S.A./N.V. Tél/Tel: + 32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. + 370 52 51 4000 |
| Magyarország Pfizer KftTel: +36 1 488 3700 |
Ceská Republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: + 35621 344610 |
Danmark Pfizer ApS Tlf.: + 45 44 201 100 | Nederland Pfizer BV Tel: +31 (0)800 63 34 636 |
Deutschland Pfizer Pharma GmbH Tel: + 49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 526 100 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel.: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H Tel: + 43 (0)1 521 15-0 |
Ελλ?δα Pfizer Ελλ?ς A.E.Τηλ.: +30 210 6785 800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Télf: +34914909900 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 1 58 07 34 40 | RomâniaPfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, LjubljanaTel.: + 386 (0) 1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL,organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf Simi: + 354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
K?προς Pfizer Ελλ?ς Α.Ε. (Cyprus Branch) Tηλ: +357 22 817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: + 371 670 35 775 |
Fecha de la última revisión de esteprospecto: 01/2025.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
-------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
La vacuna sólo debe administrarse por vía intramuscular. No administrar por vía intravascular, intradérmica o subcutánea.
Si Nimenrix se administra al mismo tiempo que otras vacunas, se deben utilizar lugares de inyección diferentes.
Nimenrix no se debe mezclar con otras vacunas.
Instrucciones para la reconstitución de la vacuna con el disolvente en jeringa precargada:
Nimenrix se debe reconstituir añadiendo todo el contenido de la jeringa precargada al vial que contiene el polvo.
Para saber cómo insertar la aguja en la jeringa, véase el dibujo explicativo. No obstante, la jeringa facilitada con Nimenrix puede ser ligeramente diferente (sin rosca de tornillo) a la jeringa descrita en el dibujo. En tal caso, la aguja deberá insertarse sin enroscar.
|
|
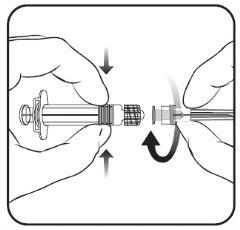
- Inserte la aguja en la jeringa
y a continuación, gírela en el sentido
de las agujas del reloj hasta que se bloquee
(ver dibujo).
- Retire el protector de la aguja;
 en algunas ocasiones puede
en algunas ocasiones puede
resultar un poco difícil.
- Añada el disolvente al polvo. Después de añadir el disolvente al polvo, debe agitar bien la mezcla hasta que el polvo esté completamente disuelto en el disolvente.
La vacuna reconstituida es una solución transparente incolora.
Se debe inspeccionar visualmente el contenido de la vacuna reconstituida para observar si existe alguna sustancia extraña y/o variación del aspecto físico antes de su administración. En caso de que se observe alguna de estas circunstancias, desechar la vacuna.
Tras la reconstitución, la vacuna debe administrarse rápidamente.
Se debe utilizar una aguja nueva para administrar la vacuna.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NIMENRIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 0,5 mlFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 0,5 mlFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 10 micrograms of Neisseria Meningitidis group A polysaccharide/dose - REVISAR µgFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para NIMENRIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NIMENRIX POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes