LUVERIS 75 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE


Cómo usar LUVERIS 75 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Disolvente en viales
Luveris 75UI polvo y disolvente para solución inyectable
lutropina alfa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamentoporque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Luveris y para qué se utiliza
- Qué necesita saber antes de empezar a usar Luveris
- Cómo usar Luveris
- Posibles efectos adversos
- Conservación de Luveris
- Contenido del envase e información adicional
1. Qué es Luveris y para qué se utiliza
Qué es Luveris
Luveris es un medicamento que contiene lutropina alfa, una Hormona Luteinizante recombinante (LH), que es esencialmente similar a la hormona que se encuentra de forma natural en los humanos, pero que se obtiene mediante biotecnología. Pertenece a la familia de hormonas llamadas gonadotropinas, que intervienen en el control normal de la reproducción.
Para qué se utiliza Luveris
Luveris se recomienda para el tratamiento de mujeres adultas que producen unas cantidades muy pequeñas de algunas hormonas implicadas en el ciclo reproductivo natural. El medicamento se utiliza junto con otra hormona llamada hormona foliculoestimulante (FSH) para provocar el desarrollo de los folículos, que son las estructuras del ovario donde maduran los huevos (óvulos). Este tratamiento se sigue de la administración de una dosis única de Gonadotropina Coriónica humana (hCG), que provoca la liberación de un óvulo desde el folículo (ovulación).
2. Qué necesita saber antes de empezar a usar Luveris
No use Luveris
- si es alérgica a las gonadotropinas (como la hormona luteinizante, la hormona foliculoestimulante o la gonadotropina coriónica humana) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si padece cáncer de ovario, de útero o de mama.
- si le han diagnosticado un tumor cerebral.
- si tiene un aumento de tamaño de los ovarios o bolsas de líquido en los ovarios (quistes ováricos) de origen desconocido.
- si tiene hemorragias vaginales inexplicadas.
No utilice Luveris si presenta alguno de los factores anteriores. Si no está segura, hable con su médico o farmacéutico antes de utilizar este medicamento.
Advertencias y precauciones
Consulte a su médico,farmacéutico o enfermero antes de empezar a usar Luveris.
Antes de iniciar el tratamiento debe valorarse tanto su fertilidad como la de su pareja.
Es recomendable que no use Luveris en caso de presentar un cuadro clínico que imposibilite un embarazo normal, como sería la situación en la que los ovarios no funcionan correctamente debido a una enfermedad denominada insuficiencia ovárica primaria o malformaciones de los órganos sexuales.
Porfiria
Antes de iniciar el tratamiento informe a su médico si usted o algún miembro de su familia padece porfiria (una incapacidad de descomponer las porfirinas que puede transmitirse de padres a hijos).
Síndrome de hiperestimulación ovárica (SHO)
Este medicamento estimula los ovarios. Esto aumenta el riesgo de que presente síndrome de hiperestimulación ovárica o SHO. Este síndrome se produce cuando sus folículos se desarrollan en exceso y se convierten en quistes de gran tamaño. Si sufre dolor abdominal, aumenta de peso en muy poco tiempo, tiene náuseas o vómitos o tiene dificultades para respirar, consulte de inmediato con su médico, quien puede indicarle que deje de usar este medicamento (ver sección 4 bajo “Efectos adversos graves”).
Si no está ovulando y se respetan la dosis y las pautas posológicas recomendadas, es menos probable que sufra SHO. El tratamiento con Luveris raramente da lugar a un SHO grave. Esto resulta más probable si también se administra el medicamento utilizado para inducir la maduración folicular final (que contiene Gonadotropina Coriónica humana, hCG). Ver sección 3 bajo “Qué cantidad se debe usar” para más datos. En caso de desarrollar un SHO, su médico puede no administrarle hCG en este ciclo de tratamiento y aconsejarle que se abstenga de realizar el coito o bien que utilice anticonceptivos de barrera durante al menos cuatro días.
Su médico asegurará un control cuidadoso de la respuesta ovárica, mediante ecografías y análisis de sangre, antes y durante el tratamiento.
Embarazo múltiple
Al usar Luveris existe un riesgo mayor de que conciba más de un bebé a la vez (“embarazo múltiple”, mayoritariamente gemelos) que si hubiera concebido de forma natural. Un embarazo múltiple puede conllevar complicaciones médicas para usted y sus bebés. Puede reducir el riesgo de embarazo múltiple utilizando la dosis correcta de Luveris en los momentos indicados. Al someterse a técnicas de reproducción asistida, el riesgo de tener un embarazo múltiple va asociado a su edad, la calidad y el número de óvulos fertilizados o a los embriones que se le implanten.
Aborto espontáneo
Al someterse a técnicas de reproducción asistida o a estimulación ovárica para producir óvulos, es más probable que se produzca un aborto espontáneo que en el promedio de las mujeres.
Embarazo ectópico
Las mujeres con antecedentes de enfermedad de las trompas de Falopio tienen riesgo de embarazo ectópico (embarazo en el que el embrión se implanta fuera del útero), independientemente de si el embarazo se consigue mediante concepción espontánea o con tratamientos de fertilidad.
Problemas de coagulación de la sangre (episodios tromboembólicos)
Consulte a su médico antes de empezar a usar Luveris si usted o algún familiar han tenido alguna vez coágulos sanguíneos en la pierna o en el pulmón, o un ataque al corazón o un ictus. Puede correr un riesgo mayor de coágulos sanguíneos graves o de que coágulos existentes se agraven al recibir tratamiento con Luveris.
Tumores de los órganos sexuales
Se han comunicado tumores en los ovarios y en otros órganos sexuales, tanto benignos como malignos, en mujeres que han recibido diversos tratamientos farmacológicos para la infertilidad.
Defectos congénitos
Los defectos congénitos tras la aplicación de técnicas de reproducción asistida pueden ser ligeramente mayores que tras embarazos espontáneos. Ello podría deberse a diferencias relacionadas con los progenitores, como la edad de la madre y los antecedentes genéticos, intervenciones relacionadas con técnicas de reproducción asistida y embarazo múltiple.
Niños y adolescentes
Luveris no está indicado para su uso en niños y adolescentes menores de 18 años de edad.
Otros medicamentos y Luveris
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No utilice Luveris mezclado con otros medicamentos en la misma inyección, excepto la folitropina alfa, si su médico la prescribe.
Embarazo y lactancia
No utilice Luveris si está embarazada o en periodo de lactancia.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
La influencia de Luveris sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Luveris contiene sodio
Luveris contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Luveris
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Uso de este medicamento
Su médico decidirá la dosis y pauta de administración más apropiadas para usted a lo largo del tratamiento.
Qué cantidad se debe usar
Luveris suele usarse diariamente, durante un máximo de tres semanas, de forma simultánea con las inyecciones de FSH.
- La dosis inicial habitual esde 75 UI (1 vial) de Luveris junto con 75 UI ó 150 UI de FSH.
- En función de la respuesta,su médico puede incrementar la dosis de FSH, preferentemente con aumentos de 37,5 a 75 UI cada 7‑14 días.
Su médico puede decidir prolongar el tratamiento hasta 5 semanas.
Cuando se haya obtenido la respuesta deseada, debe ponerse una única inyección de hCG, de 24 a 48 horas después de la última inyección de Luveris y de FSH. Se recomienda que realice usted el coito el mismo día de la administración de hCG, así como al día siguiente. Como alternativa, también puede realizarse inseminación intrauterina u otro procedimiento de reproducción médicamente asistida, a criterio de su médico.
Si se obtiene una respuesta excesiva, debe interrumpirse el tratamiento y no administrarse hCG (ver sección 4 bajo “Síndrome de hiperestimulación ovárica (SHO)”). Para el siguiente ciclo, su médico le recetará una dosis de FSH más baja que la del ciclo previo.
Forma de administración
Luveris debe administrarse por vía subcutánea, es decir, poniendo la inyección justo debajo de la piel. Cada vial es para un solo uso.
Si se administra Luveris usted misma, lea con detenimiento las siguientes instrucciones:
- Lávese las manos. Es importante que sus manos y los materiales que utilice estén lo más limpios posible.
- Reúna todo lo que vaya a necesitar. Busque un lugar limpio y prepárelo todo:
- un vial de Luveris,
- un vial de disolvente,
- dos torundas de algodón empapadas en alcohol,
- una jeringa,
- una aguja de reconstitución para disolver el polvo con el disolvente,
- una aguja fina para la inyección subcutánea,
- un recipiente para objetos cortantes para desechar de forma segura los envases de vidrio y las agujas.
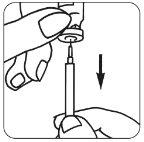
- Quite la cápsula de cierre protectora del vial de disolvente: Coloque la aguja de reconstitución
| en la jeringa e introduzca algo de aire en la jeringa tirando del émbolo aproximadamente hasta la marca de 1 ml. A continuación, introduzca la aguja en el vial, empuje el émbolo para expulsar el aire, coloque el vial boca abajo y extraiga suavemente todo el disolvente. Ponga la jeringa con cuidado sobre la mesa, procurando no tocar la aguja. |
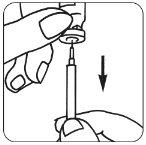
- Prepare la solución para inyección: Retire la cápsula de cierre protectora del vial que contiene
| el polvo de Luveris,coja su jeringa e inyecte lentamente el disolvente en el vial de Luveris. Muévalo suavemente sin retirar la jeringa. No lo agite. |
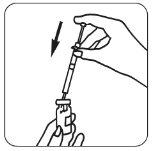
- Una vez que el polvo se haya disuelto (lo que suele ocurrir inmediatamente), compruebe que la
| solución obtenida es límpida y no contiene ninguna partícula. Ponga el vial boca abajo y extraiga suavemente la solución en la jeringa. |
Usted también puede mezclar Luveris y folitropina alfa, como alternativa a inyectar cada producto por separado. Después de disolver el polvo de Luveris, cargue la solución en la jeringa y vuelva a inyectarla en el envase que contenga el polvo de folitropina alfa. Una vez que se haya disuelto el polvo, extraiga la solución con la jeringa. Compruebe si existen partículas, como se indica más arriba, y no utilice la solución si no es límpida.
Se pueden disolver hasta 3 envases de polvo en 1 ml de disolvente.
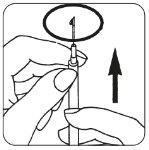
- Cambie la aguja colocando la aguja finay elimine las posibles burbujas de aire: Si ve alguna
| burbuja de aire en la jeringa, tome ésta con la aguja hacia arriba y dé golpecitos en la jeringa hasta que el aire se reúna en la parte superior. Empuje el émbolo suavemente hasta que desaparezcan las burbujas de aire. |
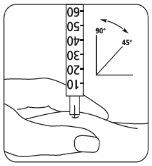
- Inyecte la solución inmediatamente: Su médico o enfermero le habrán indicado dónde debe
| poner la inyección (p. ej., vientre, parte delantera del muslo). Limpie la zona elegida con un algodón embebido de alcohol. Pellizque enérgicamente la piel e introduzca la aguja con un ángulo de 45° a 90°, con un movimiento similar al de los dardos. Inyecte bajo la piel, según las instrucciones recibidas. No inyecte directamente en una vena. Introduzca la solución presionando suavemente sobre el émbolo. Emplee todo el tiempo que necesite hasta inyectar la totalidad de la solución. Retire inmediatamente la aguja y limpie la piel con un algodón embebido de alcohol realizando un movimiento circular. |
- Deseche todo el material utilizado: Una vez finalizada la inyección, deseche inmediatamente todas las agujas y envases de vidrio vacíos en el recipiente para objetos cortantes que se suministra. Debe desecharse cualquier porción de la solución no utilizada.
Si usa más Luveris del que debe
Los efectos de una sobredosis de Luveris son desconocidos; sin embargo, existe la posibilidad de que se produzca un síndrome de hiperestimulación ovárica (ver sección 4). Sin embargo, esto sólo ocurrirá si se administra hCG (ver sección 2 bajo “Advertencias y precauciones”).
Si olvidó usar Luveris
No use una dosis doble para compensar las dosis olvidadas. Póngase en contacto con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Póngase en contacto con su médico de inmediato si advierte cualquiera de los efectos adversos enumerados a continuación. El médico podría indicarle que dejara de usar Luveris.
Reacción alérgica
Las reacciones alérgicas tales como erupción cutánea, enrojecimiento de la piel, ampollas e inflamación de la cara con dificultad para respirar pueden ser graves en ciertos casos. Este efecto adverso es muy raro (puede afectar hasta 1 de cada 10 000 personas).
Síndrome de hiperestimulación ovárica (SHO)
- El dolor en la región inferior del abdomen acompañado de náuseas o vómitos pueden ser síntomas del síndrome de hiperestimulación ovárica (SHO). Los ovarios pueden haber reaccionado de forma excesiva al tratamiento y pueden haber formado grandes bolsas de líquido o quistes (ver sección 2 bajo “Síndrome de hiperestimulación ovárica (SHO)”). Este efecto adverso es frecuente (puede afectar hasta 1 de cada 10 personas). Si ocurre esto, su médico tendrá que examinarla lo antes posible.
- En casos muy raros se dan complicaciones graves de coagulación sanguínea (episodios tromboembólicos), por lo general con SHO grave. Esto puede causar dolor en el pecho, falta de aliento, ictus o ataque al corazón (ver sección 2 bajo “Problemas de coagulación de la sangre”).
Otros efectos secundarios frecuentes
- Dolor de cabeza
- Náuseas, vómitos, diarrea, molestias abdominales o dolor abdominal
- Bolsas de líquido en los ovarios (quistes ováricos), dolor en el pecho y dolor pélvico
- Reacciones locales en el lugar de inyección, como son dolor, picor, hematomas, inflamación o irritación
No se ha comunicado ningún caso de torsión del ovario ni hemorragias en el abdomen tras el tratamiento con Luveris; sin embargo, se han comunicado casos raros tras el tratamiento con gonadotropina menopáusica humana (hMG), un medicamento obtenido a partir de la orina, que también contiene LH.
Puede producirse embarazo ectópico (implantación del embrión fuera del útero), especialmente en mujeres con antecedentes de enfermedades de las trompas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Luveris
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en los viales después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25 °C. Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento si observa indicios visibles de deterioro, tales como decoloración del polvo o daño del envase.
El medicamento debe administrarse inmediatamente después de disolver el polvo.
La solución no debe administrarse si contiene partículas o no es límpida.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Luveris
- El principio activo es lutropina alfa. Un vial de polvo inyectable contiene 75 UI (Unidades Internacionales).
- La lutropina alfa es Hormona Luteinizante humana recombinante, r-hLH, producida mediante tecnología de ADN recombinante.
- Los demás componentes del polvo son polisorbato 20, sacarosa, fosfato monosódico monohidrato, fosfato disódico dihidrato, ácido fosfórico concentrado, hidróxido sódico, L‑metionina y nitrógeno.
- El disolvente es agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
- Luveris se presenta como un polvo y disolvente para solución inyectable.
- Cada vial de polvo contiene 75 UI de lutropina alfa y cada vial de disolvente contiene 1 ml de agua para preparaciones inyectables.
- Luveris se presenta en cajas que contienen 1, 3 ó 10 viales de polvo junto con el mismo número de viales de disolvente.
Titular de la autorización de comercialización
Merck Europe B.V.
Gustav Mahlerplein 102
1082 MA Amsterdam
Países Bajos
Responsable de la fabricación
Merck Serono S.p.A.
Via delle Magnolie 15
70026 Modugno (Bari)
Italia
Fecha de la última revisión deeste prospecto:06/2022
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LUVERIS 75 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150 UI/ 0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere recetaForma farmacéutica: INYECTABLE, 150UI/0,25 ml (11 microgramos/ 0,25 ml)Principio activo: Follitropin alfaFabricante: Gedeon Richter Plc.Requiere receta
Médicos online para LUVERIS 75 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LUVERIS 75 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes