
ЛЕМТРАДА 12 мг концентрат для приготування розчину для інфузій


Інструкція із застосування ЛЕМТРАДА 12 мг концентрат для приготування розчину для інфузій
Вступ
Опис: інформація для пацієнта
LEMTRADA12мг концентрат для розчину для інфузії
алемтузумаб
Цей лікарський засіб підлягає додатковому моніторингу, що прискорить виявлення нової інформації про його безпеку. Ви можете допомогти, повідомивши про будь-які побічні ефекти, які ви можете мати. Остання частина розділу 4 містить інформацію про те, як повідомляти про ці побічні ефекти.
Вважно прочитайте весь опис уважно перед тим, як вам буде введено цей лікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке LEMTRADA і для чого він використовується
- Що вам потрібно знати перед тим, як вам буде введено LEMTRADA
- Як буде введено LEMTRADA
- Можливі побічні ефекти
- Збереження LEMTRADA
- Зміст упаковки та додаткова інформація
1. Що таке LEMTRADA і для чого він використовується
LEMTRADA містить активну речовину алемтузумаб, який використовується для лікування однієї форми розсіяного склерозу (РС) у дорослих, яка називається рецидивно-ремітентним розсіяним склерозом (РРРС). LEMTRADA не лікує РС, але може зменшити кількість рецидивів. Також він може сповільнити або повернути деякі ознаки та симптоми РС. У клінічних дослідженнях пацієнти, які отримували LEMTRADA, мали менше рецидивів і менш ймовірно мали погіршення інвалідності порівняно з пацієнтами, які отримували інтерферон бета, введений кілька разів на тиждень.
LEMTRADA використовується, якщо ваш РС дуже активний, незважаючи на те, що ви вже були-treated з至少 одним іншим лікарським засобом для РС, або якщо ваш РС швидко прогресує.
Що таке розсіяний склероз?
РС - це аутоімунне захворювання, яке впливає на центральну нервову систему (мозок і спинний мозок). У РС імунна система атакує помилково захисну оболонку (мієлін), яка покриває нервові волокна, що призводить до запалення. Коли запалення викликає симптоми, його зазвичай називають "атакою" або "рецидивом". У РРРС пацієнти переживають рецидиви, за якими слідують періоди відновлення.
Симптоми, які виникають, залежать від частини центральної нервової системи, яка уражена. Ушкодження, яке викликається нервами під час цього запалення, може бути оборотнім, але чим більше прогресує захворювання, тим більше ушкодження можуть накопичуватися і ставати постійними.
Як працюєLEMTRADA
LEMTRADA регулює імунну систему для обмеження її атак на нервову систему.
2. Що потрібно знати перед тим, як буде введено ЛЕМТРАДА
НЕ використовуйте ЛЕМТРАДУ:
- якщо ви є алергічні на алемтузумаб або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6)
- якщо ви є носієм вірусу імунодефіциту людини (ВІЛ)
- якщо у вас є тяжка інфекція
- якщо у вас є будь-яка з наступних хвороб:
- інша автоімунна хвороба, окрім множинного склерозу
- гіпертонія, що не піддається контролю
- анамнез розриву судин, що постачають кров'ю мозок
- анамнез інсульту (інсульту)
- анамнез інфаркту міокарда або стенокардії
- анамнез геморагічного розладу.
Попередження та застереження
Порадьтеся з вашим лікарем перед тим, як буде введено ЛЕМТРАДА. Після закінчення курсу лікування ЛЕМТРАДОЮ ви можете мати підвищений ризик розвитку інших автоімунних хвороб або переживання тяжких інфекцій. Важливо, щоб ви розуміли ці ризики та знали, як їх виявити. Вам буде видана Карта пацієнта та Посібник для пацієнта з більшою кількістю інформації. Важливо, щоб ви мали Карту пацієнта з собою під час лікування та протягом 4 років після останньої інфузії ЛЕМТРАДИ, оскільки можуть виникнути побічні ефекти через роки після лікування. Коли ви перебуваєте під медичним наглядом, хоча б не для РМС, покажіть Карту пацієнта лікареві.
Ваш лікар проведе аналізи крові перед тим, як буде розпочато лікування ЛЕМТРАДОЮ. Ці аналізи проводяться для того, щоб дізнатися, чи можете ви приймати ЛЕМТРАДУ. Ваш лікар також переконається, що у вас немає певних хвороб або медичних розладів перед тим, як буде розпочато лікування ЛЕМТРАДОЮ.
- Автоімунні хвороби
Лікування ЛЕМТРАДОЮ може збільшити ризик автоімунних хвороб. Ці хвороби характеризуються тим, що імунна система атакує, помилково, власне тіло. Нижче надається інформація про деякі конкретні хвороби, які були спостережені у пацієнтів з РМС, які були лікувані ЛЕМТРАДОЮ.
Автоімунні хвороби можуть виникнути через багато років після лікування ЛЕМТРАДОЮ. Тому необхідно проводити аналізи крові та сечі протягом 4 років після останньої інфузії. Аналізи необхідні, навіть якщо ви відчуваєте себе добре і симптоми РМС під контролем. Є певні ознаки та симптоми, на які ви повинні звернути увагу. Крім того, ці хвороби можуть виникнути після закінчення 4 років, тому вам потрібно бути уважним до ознак та симптомів, навіть після того, як вже не потрібно проводити щомісячні аналізи крові та сечі. У розділах 2 та 4 – автоімунні хворобинадаються подробиці про ознаки та симптоми, аналізи та дії, які ви повинні вчинити.
У посібнику для пацієнта ЛЕМТРАДИможна знайти більше корисної інформації про ці автоімунні хвороби (та пов'язані з ними тести).
- Гемофілія А, набута
Рідко пацієнти розвивали геморагічний розлад, викликаний антитілами, що діють проти фактору VIII (білка, необхідної для нормального згортання крові), звану набутою гемофілією А. Ця хвороба повинна бути діагностована та лікувана негайно. Симптоми набутої гемофілії А описані в розділі 4.
- Імунна тромбоцитопенічна пурпура (ІТП)
Часто пацієнти розвивали геморагічний розлад, викликаний низьким рівнем тромбоцитів, званий імунною тромбоцитопенією (ІТП). Цей розлад повинен бути виявлений та лікувався швидко, оскільки в іншому випадку його ефекти можуть бути тяжкими або навіть смертельними. Ознаки та симптоми ІТП описані в розділі 4.
- Ниркова хвороба (наприклад, хвороба анти-МБГ)
Рідко пацієнти мали проблеми, пов'язані з автоімунними розладами в нирках, наприклад, хворобу анти-мембранної базальної гломерули (хворобу анти-МБГ). Ознаки та симптоми ниркової хвороби описані в розділі 4. Якщо не лікувати, це може призвести до ниркової недостатності з необхідністю діалізу або трансплантації та може спричинити смерть.
- Розлади щитоподібної залози
Часто пацієнти мали автоімунний розлад щитоподібної залози, який впливає на її здатність виробляти або контролювати важливі для метаболізму гормони.
ЛЕМТРАДА може викликати різні типи розладів щитоподібної залози, включаючи:
- Гіперактивна щитоподібна залоза(гіпертиреоз) при надмірній продукції гормонів
- Гіпоактивна щитоподібна залоза(гіпотиреоз) при недостатній продукції гормонів
Ознаки та симптоми розладів щитоподібної залози описані в розділі 4.
Якщо ви розвиваєте розлад щитоподібної залози, у більшості випадків вам потрібно буде лікувати протягом всього життя препаратами, які контролюють розлад, та в деяких випадках може бути необхідне видалення щитоподібної залози.
Важливо слідувати належному лікуванню розладу щитоподібної залози, особливо якщо ви вагітні після лікування ЛЕМТРАДОЮ. Нелікований розлад щитоподібної залози може завдати шкоди плоду або новонародженому.
- Запалення печінки
Деякі пацієнти мали запалення печінки після прийому ЛЕМТРАДИ. Запалення печінки можна діагностувати за допомогою аналізів крові, які проводяться регулярно після лікування ЛЕМТРАДОЮ. Повідомте вашому лікареві, якщо у вас є один або декілька з наступних симптомів: нудота, блювота, біль у животі, втома, зниження апетиту, жовтяниця шкіри або очей, темна сеча або кровотеча чи синяки з більшою легкістю, ніж зазвичай.
- Тромботична тромбоцитопенічна пурпура (ТТП)
ЛЕМТРАДА може викликати розлад згортання крові, званий тромботичною тромбоцитопенією (ТТП). Кров'яні згустки утворюються в судинах та можуть виникнути в усьому тілі. Надішліть негайну медичну допомогу, якщо у вас є один з наступних симптомів: синяки на шкірі або в роті, які можуть виглядати як червоні точки, з або без надмірної втоми, гарячки, змін у мовленні, жовтяниці шкіри або очей (жовтяниця), зниження кількості сечі, темна сеча. Рекомендується шукати негайну медичну допомогу, оскільки тривале існування ТТП може бути смертельним (див. розділ 4 "Можливі побічні ефекти").
- Саркоїдоз
У пацієнтів, лікуваних ЛЕМТРАДОЮ, було повідомлено про розлад імунної системи (саркоїдоз). Симптоми можуть включати сухий кашель, задишку, біль у грудях, гарячку, запалення лімфатичних вузлів, втрату ваги, шкірні висипи та розмитість зору.
- Автоімунний енцефаліт
Автоімунний енцефаліт (імуномедіований розлад мозку) може виникнути після прийому ЛЕМТРАДИ. Ця хвороба може включати симптоми, такі як зміни поведінки та/або психічні розлади, втрата пам'яті на короткий термін або конвульсії. Симптоми можуть бути подібними до рецидиву РМС. Якщо ви розвиваєте один або декілька з цих симптомів, зверніться до вашого лікаря.
- Інші автоімунні хвороби
Рідко деякі пацієнти мали автоімунні хвороби, пов'язані з червоними кров'яними тілецьками або білими кров'яними тілецьками. Ці хвороби можна діагностувати за допомогою аналізів крові, які проводяться регулярно після лікування ЛЕМТРАДОЮ. Якщо ви розвиваєте одну з цих хвороб, ваш лікар повідомить вам про це та прийме необхідні заходи для лікування.
- Реакції на інфузію
Більшість пацієнтів, лікуваних ЛЕМТРАДОЮ, мали побічні ефекти під час інфузії або протягом 24 годин після неї. Для зменшення реакцій на інфузію лікар лікуватиме вас іншими препаратами (див. розділ 4 – реакції на інфузію).
- Інші тяжкі реакції відбуваються незабаром після інфузії ЛЕМТРАДИ
Деякі пацієнти мали тяжкі або потенційно смертельні реакції після інфузії ЛЕМТРАДОЮ, включаючи кровотечу в легенях, інфаркт міокарда, інсульт (інсульт) або розрив судин, що постачають кров'ю мозок. Реакції можуть виникнути після будь-якої з доз під час курсу лікування. У більшості випадків реакції відбулися протягом 1-3 днів після інфузії. Ваш лікар контролюватиме ваші життєві показники, включаючи артеріальний тиск, перед та під час інфузії. Надішліть негайну допомогу, якщо у вас є один з наступних симптомів: труднощі з диханням, кашель з кров'ю, біль у грудях, опущення обличчя, раптовий сильний головний біль, слабкість у одному боці тіла, труднощі з мовленням або біль у шиї.
- Лімфогістіоцитоз гемофагоцитарний
Лікування ЛЕМТРАДОЮ може збільшити ризик надмірної активації білих кров'яних тілець, пов'язаних з запаленням (лімфогістіоцитоз гемофагоцитарний), який може бути смертельним, якщо не діагностувати та лікувати вчасно. Якщо ви маєте декілька симптомів, таких як гарячка, запалення лімфатичних вузлів, синяки або шкірні висипи, зверніться до вашого лікаря негайно.
- Хвороба Стілла у дорослих (ХСД)
ХСД – це рідка хвороба, яка може викликати запалення в декількох органах з різними симптомами, такими як гарячка >39 °C або 102,2 °F, яка триває більше 1 тижня, біль,僵кість з або без запалення в декількох суглобах та/або шкірні висипи. Якщо ви маєте комбінацію цих симптомів, зверніться до вашого лікаря негайно.
- Інфекції
Пацієнти, лікувані ЛЕМТРАДОЮ, мають підвищений ризик розвитку тяжкої інфекції(див. розділ 4 – інфекції). Загалом, інфекції можна лікувати стандартними препаратами.
Для зменшення ризику інфекції ваш лікар перевірить, чи інші препарати, які ви приймаєте, можуть впливати на вашу імунну систему. Через це важливо повідомити лікареві про всі препарати, які ви приймаєте.
Також повідомте вашому лікареві, якщо у вас є тяжка інфекція перед тим, як буде розпочато лікування ЛЕМТРАДОЮ, оскільки ваш лікар повинен відкласти лікування до тих пір, поки інфекція не буде ліквідована.
Пацієнти, лікувані ЛЕМТРАДОЮ, мають підвищений ризик розвитку інфекції, викликаної герпесвірусом (наприклад, стоматит). Загалом, якщо пацієнт мав інфекцію, викликану герпесвірусом, він має підвищений ризик розвитку іншої інфекції. Також можливо розвиток інфекції, викликаної герпесвірусом, вперше. Рекомендується, щоб лікар призначив відповідне лікування для зменшення ризику розвитку інфекції, викликаної герпесвірусом, яке потрібно приймати протягом днів, коли проводиться лікування ЛЕМТРАДОЮ, та протягом місяця після лікування.
Крім того, можуть виникнути інфекції, які можуть викликати анормальності шийки матки(цервікс). Через це рекомендується, щоб усі пацієнтки проводили щорічний огляд, типу цервікального мазка. Ваш лікар повідомить вам, які тести вам потрібно проводити.
Було повідомлено про інфекції, викликані вірусом, званим цитомегаловірус, у пацієнтів, лікуваних ЛЕМТРАДОЮ. Більшість випадків відбулися протягом 2 місяців після введення алемтузумабу. Повідомте вашому лікареві негайно, якщо у вас є симптоми інфекції, такі як гарячка або запалення лімфатичних вузлів.
Пацієнти, лікувані ЛЕМТРАДОЮ, мали інфекції, викликані вірусом, званим вірус Епштейна-Барра (ВЕБ), включаючи випадки з тяжким запаленням печінки та іноді смертельними. Повідомте вашому лікареві негайно, якщо у вас є симптоми інфекції, такі як гарячка, запалення лімфатичних вузлів або втома.
Пацієнти, лікувані ЛЕМТРАДОЮ, також мають підвищений ризик розвитку інфекції, викликаної лістерією(бактеріальна інфекція, викликана вживанням забруднених продуктів). Інфекція, викликана лістерією, може викликати тяжку хворобу, включаючи менінгіт, але її можна лікувати відповідними препаратами. Для зменшення цього ризику рекомендується уникати вживання сирого м'яса або недостатньо приготовленого м'яса, свіжих сирів та молочних продуктів, які не були пастеризовані, протягом 2 тижнів до початку лікування, під час лікування та протягом至少 1 місяця після лікування ЛЕМТРАДОЮ.
Якщо ви живете в районі, де часто зустрічається туберкульоз, ви можете мати підвищений ризик захворювання на нього. Ваш лікар призначить тести для виявлення туберкульозу.
Якщо ви є носієм вірусу гепатиту Б або гепатиту С(які впливають на печінку), потрібно бути обережним перед тим, як буде введено ЛЕМТРАДА, оскільки невідомо, чи лікування може призвести до активації інфекції, яка згодом може пошкодити печінку.
Було повідомлено про випадки рідкої інфекції мозку, званої ЛМП (леукоенцефалопатія, мультифокальна, прогресивна), у пацієнтів, які приймали Лемтраду. ЛМП була повідомлена у пацієнтів з іншими факторами ризику, зокрема попереднім лікуванням препаратами проти РМС, пов'язаними з ЛМП.
ЛМП може призвести до важкої інвалідності протягом тижнів або місяців та може бути смертельною.
Симптоми можуть бути подібними до рецидиву РМС та включають прогресивну слабкість або незграбність кінцівок, порушення зору, труднощі з мовленням або зміни у мисленні, пам'яті та орієнтації, які викликають розгубленість та зміни особистості. Важливо повідомити вашу сім'ю чи опікунів про ваше лікування, оскільки вони можуть помітити симптоми, яких ви не усвідомлюєте. Повідомте вашому лікареві негайно, якщо у вас є будь-який симптом, який може бути ознакою ЛМП.
- Пневмоніт та перикардит
У пацієнтів, лікуваних ЛЕМТРАДОЮ, було повідомлено про пневмоніт (запалення легеневого тканини). Більшість випадків відбулися протягом першого місяця після лікування ЛЕМТРАДОЮ. Також було повідомлено про випадки перикардіального випоту (накопичення рідини навколо серця) та перикардиту (запалення мембрани, яка оточує серце) у пацієнтів, лікуваних ЛЕМТРАДОЮ. Повідомте вашому лікареві про симптоми, такі як труднощі з диханням, кашель, свист, біль або тиснява в грудях, кровотеча при кашлі, оскільки ці симптоми можуть бути викликані пневмонитом, перикардіальним випотом або перикардитом.
- Запалення жовчного міхура
ЛЕМТРАДА може збільшити ризик запалення жовчного міхура. Це може бути важка медична хвороба, яка може бути смертельною. Повідомте вашому лікареві, якщо у вас є симптоми, такі як біль у животі або нездужання, гарячка, нудота або блювота.
- Попередній діагноз раку
Якщо вам було діагностовано рак раніше, повідомте вашому лікареві.
- Вакцинація
Невідомо, чи ЛЕМТРАДА впливає на реакцію на вакцинацію. Якщо ви не завершили стандартну вакцинацію, лікар вирішить, чи потрібно вам зробити вакцинацію перед тим, як буде розпочато лікування ЛЕМТРАДОЮ. Зокрема, ваш лікар розгляне можливість вакцинації проти вітряної віспи, якщо ви ще не мали її. Будь-яку вакцинацію потрібно проводити за至少 6 тижнів до початку курсу лікування ЛЕМТРАДОЮ.
Ви НЕ повинні отримувати певні типи вакцин (вакцини з живими вірусами) якщо ви приймали ЛЕМТРАДУ недавно.
Діти та підлітки
ЛЕМТРАДА не повинна бути використана у дітей та підлітків молодше 18 років, оскільки її не вивчали у пацієнтів з РМС молодше цього віку.
Інші препарати та ЛЕМТРАДА
Повідомте вашому лікареві або фармацевту, якщо ви приймаєте, приймали недавно або можете приймати будь-який інший препарат (включаючи вакцинацію або препарати на основі рослин).
Окрім ЛЕМТРАДИ, існують інші методи лікування (включаючи ті, які використовуються для лікування РМС або інших хвороб), які можуть впливати на вашу імунну систему та, таким чином, впливати на вашу здатність захищатися від інфекцій. Якщо ви використовуєте будь-який з цих препаратів, ваш лікар може попросити вас припинити їх приймати перед тим, як буде розпочато лікування ЛЕМТРАДОЮ.
Вагітність
Якщо ви вагітні, думаєте, що можете бути вагітною або плануєте вагітність, проконсультуйтеся з вашим лікарем перед тим, як буде введено цей препарат.
Жінки репродуктивного віку повинні використовувати ефективні методи контрацепції під час кожного курсу лікування ЛЕМТРАДОЮ та протягом 4 місяців після кожного курсу.
Якщо ви вагітні після лікування ЛЕМТРАДОЮ та маєте розлад щитоподібної залози під час вагітності, потрібно бути особливо обережним. Нелікований розлад щитоподібної залози може завдати шкоди плоду.
Грудне вигодовування
Невідомо, чи ЛЕМТРАДА виділяється в грудне молоко, але існує можливість цього. Рекомендується перервати грудне вигодовування під час кожного курсу лікування ЛЕМТРАДОЮ та протягом 4 місяців після кожного курсу лікування. Однак грудне молоко може бути корисним (може захищати дитини від інфекцій), тому проконсультуйтеся з вашим лікарем, якщо плануєте годувати грудьми вашу дитину, і він порадить вам, що краще для вас та вашої дитини.
Фертильність
ЛЕМТРАДА може залишатися в вашому організмі протягом курсу лікування та до 4 місяців після нього. Невідомо, чи ЛЕМТРАДА має ефекти на фертильність протягом цього періоду. Проконсультуйтеся з вашим лікарем, якщо плануєте мати дітей. Немає доказів того, що ЛЕМТРАДА має вплив на фертильність чоловіків.
Водіння та використання машин
Багато пацієнтів мають побічні ефекти під час інфузії ЛЕМТРАДОЮ або протягом 24 годин після неї, і деякі з цих ефектів, наприклад, запаморочення, можуть зробити водіння або використання машин небезпечним. Якщо це відбувається з вами, перервіть ці діяльності до тих пір, поки ви не почуватиметеся краще.
ЛЕМТРАДАмістить калій та натрій
Цей препарат містить калій, менше 1 ммоль калію(39 мг) на інфузію; це означає, що він "практично не містить калію".
Цей препарат містить менше 1 ммоль натрію(23 мг) на інфузію; це означає, що він "практично не містить натрію".
3. Як буде застосовуватися ЛЕМТРАДА
Ваш лікар пояснить, як застосовується ЛЕМТРАДА. У разі сумнівів запитайте у вашого лікаря.
Початкове лікування, яке ви отримаєте, складатиметься з інфузії один раз на день протягом 5 днів (цикл 1) та через рік - інфузії один раз на день протягом 3 днів (цикл 2).
Між двома циклами ви не будете отримувати лікування ЛЕМТРАДОЮ. Два цикли лікування можуть зменшити активність РС до 6 років.
Деяким пацієнтам, якщо у них з'являються симптоми або ознаки захворювання РС після двох початкових циклів, можуть призначити додатково один або два цикли лікування, які складаються з інфузії один раз на день протягом 3 днів. Ці додаткові цикли лікування можна призначити через дванадцять місяців або пізніше після попереднього лікування.
Максимальна добова доза становить одну інфузію.
ЛЕМТРАДА буде введена вам через вену. Кожна інфузія триває близько 4 годин. Нагляд за виявленням побічних ефектів та періодичні тести повинні продовжуватися протягом 4 років після останньої інфузії.
Щоб допомогти вам краще зрозуміти тривалість дії лікування та тривалість необхідного нагляду, зверніться до наступної схеми.
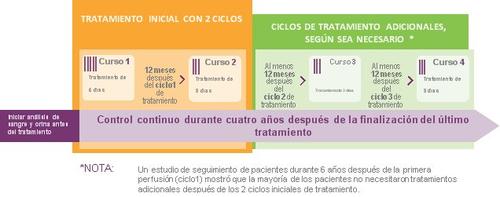
Нагляд після лікування ЛЕМТРАДОЮ
Після того, як ви отримаєте ЛЕМТРАДУ, вам потрібно буде проходити періодичні тести, щоб будь-який можливий побічний ефект можна було діагностувати та лікувати швидко. Ці тести повинні продовжуватися протягом 4 років після останньої інфузії та описані в розділі 4, найважливіші побічні ефекти.
Якщо ви отримаєте більше ЛЕМТРАДИ, ніж потрібно
Пацієнти, яким випадково було введено надто багато ЛЕМТРАДИ під час інфузії, trảiли серйозні реакції, такі як головний біль, висип, низький кров'яний тиск або збільшення частоти серцевих скорочень. Вищі дози, ніж рекомендовані, можуть спричинити більш серйозні або тривалі реакції на інфузію (див. розділ 4) або більший вплив на імунну систему. Лікування складається з припинення введення ЛЕМТРАДИ та лікування симптомів.
Якщо ви забули використати ЛЕМТРАДУ
Мало ймовірно, що ви забудете про свою дозу, оскільки її вводить медичний працівник. Однак пам'ятайте, що якщо ви пропустите дозу, її не слід вводити в той же день, що й запланована доза.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, запитайте у вашого лікаря.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може спричиняти побічні ефекти, хоча не всі люди їх trảiли.
Найважливіші побічні ефекти- це автоімунні захворювання, описані в розділі 2, серед яких:
- Гемофілія А, набута(рідкісний побічний ефект, який може впливати до 1 людини з 100): може проявлятися у вигляді спонтанних синяків, носових кровотеч, болю чи запалення суглобів, інших видів кровотеч або кровотеч при порізі, які можуть тривати довше, ніж зазвичай.ПТІ (геморагічний розлад),(частий побічний ефект - може впливати до 1 людини з 10): може проявлятися у вигляді малих червоних, рожевих або фіолетових плям, розсіяних по шкірі; схильність до синяків; кровотечі при порізі, які важче зупинити; менструальні періоди, які тривають довше чи частіше, ніж зазвичай; кровотечі між менструальними періодами; нові або триваліші, ніж зазвичай, кровотечі з ясен чи носа; або кашель з кров'ю.
- Пурпура тромботична тромбоцитопенічна (ПТТ), (рідкісний побічний ефект - може впливати до 1 людини з 1000): може проявлятися у вигляді синяків на шкірі чи у роті, які можуть виглядати як червоні точки, з або без надмірної втоми, лихоманки, сплутаності, змін у мовленні, жовтого забарвлення шкіри чи очей (жовтяниця), низької кількості сечі, темної сечі.
- Ниркові розлади, (рідкісний побічний ефект - може впливати до 1 людини з 1000): можуть проявлятися у вигляді крові в сечі (сеча може бути червоною чи кольору чаю) або у вигляді набухання ніг чи ступнів. Також можуть спричиняти ушкодження легень та спричиняти кашель з кров'ю.
Якщо ви trảiли будь-які з цих ознак чи симптомів, пов'язаних з нирковими чи геморагічними розладами, негайно повідомте своєму лікареві. Якщо ви не можете зв'язатися зі своїм лікарем, шукайте негайної медичної допомоги.
- Тиреоїдні розлади(бардzo частий побічний ефект - може впливати понад 1 людини з 10): можуть проявлятися у вигляді надмірної потовиділення; збільшення чи зниження ваги без пояснення; набухання очей; нервозності; прискореного серцевого ритму; відчуття холоду; погіршення втоми; або нової запори.
- Розлади червоних та білих кров'яних тілець(рідкісний побічний ефект - може впливати до 1 людини з 100) діагностовані за допомогою аналізів крові.Саркоїдоз(рідкісний побічний ефект - може впливати до 1 людини з 100): симптоми можуть включати у себе сухий кашель, коротке дихання, грудний біль, лихоманку, набухання лімфатичних вузлів, втрату ваги, шкірні висипи та розмиту зір.
- Автоімунний енцефаліт(рідкісний побічний ефект - може впливати до 1 людини з 100): можуть включати симптоми, такі як зміни поведінки та/або психічні розлади, короткочасна втрата пам'яті чи конвульсії. Симптоми можуть нагадувати рецидив РС.
Всі ці серйозні побічні ефекти можуть з'явитися через роки після введення ЛЕМТРАДИ. Якщо ви trảiли будь-які з цих ознак чи симптомів, негайно повідомте своєму лікареві.Також вам будуть проводити періодичні аналізи крові та сечі, щоб забезпечити, що якщо ви розвинете одну з цих захворювань, її можна буде лікувати швидко.
Перегляд тестів, які будуть проведені для контролю появи автоімунних станів:
Тест | Коли? | Тривалість |
Аналіз крові (для діагностики всіх важливих серйозних побічних ефектів, згаданих вище) | До початку лікування та кожний місяць після лікування | До 4 років після останньої інфузії ЛЕМТРАДИ |
Аналіз сечі (додатковий тест для діагностики ниркових розладів) | До початку лікування та кожний місяць після закінчення лікування | До 4 років після останньої інфузії ЛЕМТРАДИ |
Після цього періоду, якщо ви trảiли симптоми ПТІ, гемофілії А, набутої, ПТТ, тиреоїдних чи ниркових розладів, ваш лікар проведе додаткові тести. Ви повинні продовжувати бути уважними до ознак і симптомів побічних ефектів після чотирьох років, як вказано в Посібництві для пацієнта, та продовжувати носити з собою Карточку пацієнта.
Інший важливий побічний ефект- це збільшення ризику інфекцій(див. нижче інформацію про частоту, з якою пацієнти trảiли інфекції). У більшості випадків ці інфекції є легкими, але можуть спричиняти серйозні інфекції.
Якщо ви trảiли будь-які з цих ознак інфекції,негайно повідомте своєму лікареві
|
Щоб допомогти вам зменшити ризик деяких інфекцій, ваш лікар може розглянути можливість проведення вакцинації проти вітряної віспи та/або інших вакцинацій, які він вважає необхідними (див. розділ 2: Що вам потрібно знати перед початком використання ЛЕМТРАДИ- Вакцинація). Ваш лікар також може призначити лікарський засіб для лікування виразок у роті (див. розділ 2: Що вам потрібно знати перед початком використання ЛЕМТРАДИ- Інфекції).
Найбільш часті побічні ефекти- це реакції на інфузію(див. нижче інформацію про частоту, з якою пацієнти trảiли ці реакції), які можуть виникнути під час інфузії або протягом 24 годин після неї. У більшості випадків ці реакції є легкими, але можуть бути серйозними. Іноді можуть виникнути алергічні реакції.
Щоб спробувати зменшити реакції на інфузію, лікар введе вам лікарські засоби (кортикостероїди) до кожної з перших трьох інфузій циклу ЛЕМТРАДИ. Щоб обмежити ці реакції, також можуть бути призначені інші методи лікування до інфузії чи при виникненні симптомів. Крім того, вас будуть спостерігати під час інфузії та до 2 годин після її закінчення. У разі серйозних реакцій інфузію можна сповільнити або навіть зупинити.
Зверніться до Посібництва для пацієнта ЛЕМТРАДИдля отримання більшої інформації про ці події.
Це побічні ефекти, які ви можете trảiти:
Бардzo часті(можуть впливати понад 1 людини з 10):
- Реакції на інфузію, які можуть виникнути під час інфузії або протягом 24 годин після неї: зміни серцевого ритму, головний біль, висип, висип по всьому тілу, лихоманка, кропив'янка, озноб, свербіж, червоне забарвлення обличчя та шиї, відчуття втоми, нудота
- Інфекції: інфекції дихальної системи, такі як застуда та синусит, інфекції сечовидільної системи, інфекції, викликані герпесом
- Зниження кількості білих кров'яних тілець (лімфоцитів, лейкоцитів, нейтрофілів)
- Тиреоїдні розлади, такі як гіпер- чи гіпотиреоз
Часті(можуть впливати до 1 людини з 10):
- Реакції на інфузію, які можуть виникнути під час інфузії або протягом 24 годин після неї: диспепсія, грудний біль, біль, головокружіння, порушення смаку, труднощі зі сном, труднощі з диханням чи коротке дихання, низький кров'яний тиск, біль у місці інфузії
- Інфекції: кашель, інфекція вуха, грипоподібне захворювання, бронхіт,пневмонія, вагінальний кандидоз, герпес, виразки у роті, набухання залоз чи збільшення їхнього розміру, грип, інфекція, викликана герпесвірусом зостера, інфекція зубів
- Збільшення кількості білих кров'яних тілець, таких як нейтрофіли, еозінофіли (різні типи білих кров'яних тілець), анемія, зниження проценту червоних кров'яних тілець, підвищена схильність до синяків чи кровотеч, набухання лімфатичних вузлів
- Надмірна імунна відповідь
- Біль у спині, шиї, руках чи ногах, м'язовий біль, м'язові спазми, артралгія, біль у роті чи горлі
- Набухання рота/ясен/мови
- Загальне нездужання, слабкість, блювота, діарея, абдомінальний біль, кишкова грип, ікота
- Аномальний аналіз печінки
- Пальпитація серця
- Аномалії, які можуть бути виявлені під час огляду: кров чи білок у сечі, знижений серцевий ритм, нерегулярний чи аномальний серцевий ритм, високий кров'яний тиск, порушення ниркової функції, білі кров'яні тілець у сечі
- Синяк
- Рецидив РС
- Тремор, втрата чутливості, відчуття печіння чи поколювання
- Гіпер- чи гіпотиреоз, автоімунний за походженням, тиреоїдні антитіла чи зоб (набухання тиреоїдної залози шиї)
- Оніміння рук чи ніг
- Проблеми з зором, кон'юнктивіт, захворювання очей, пов'язані з захворюванням тиреоїдної залози
- Чувство головокружіння чи втрати рівноваги, мігрень
- Чувство тривоги, депресія
- Нерегулярні, тривалі чи надмірні менструації
- Акне, червоне забарвлення шкіри, надмірна потовиділення, зміна кольору шкіри, ушкодження шкіри, дерматит
- Носові кровотечі, синяки
- Випадіння волосся
- Астма
- М'язовий та кістковий біль, загальне нездужання у грудній клітці
Рідкісні(можуть впливати до 1 людини з 100):
- Інфекції: кишкова грип, гінгівіт, грибкова інфекція нігтів, тонзиліт, гострий синусит, бактеріальна інфекція шкіри, цитомегаловірусна інфекція
- Пневмоніт
- Грибкова інфекція ніг
- Аномальний вагінальний мазок
- Збільшення чутливості, порушення чутливості, такі як оніміння, поколювання та біль, напружений головний біль
- Двоєрідність зору
- Вушний біль
- Труднощі з ковтанням, подразнення горла, продуктивний кашель
- Зниження ваги, збільшення ваги, зниження кількості червоних кров'яних тілець, підвищення рівня глюкози в крові, збільшення розміру червоних кров'яних тілець
- Запор, кислотний рефлюкс, сухість у роті
- Кровотеча з прямої кишки
- Кровотеча з ясен
- Зниження апетиту
- Пухирці, нічні поти, набухання обличчя, екзема
- Ригідність, загальне нездужання у руках чи ногах
- Камені в нирках, виділення кетонових тіл у сечі, ниркове захворювання
- Зниження/ослаблення імунної системи
- Туберкульоз
- Набухання жовчного міхура з чи без каменів у ньому (жовчові камені)
- Кондиломи
- Автоімунний розлад, характеризований кровотечею (гемофілія А, набута)
- Саркоїдоз
- Автоімунний розлад мозку (автоімунний енцефаліт)
- Плями на шкірі з втратою пігменту (вітіліго)
- Випадіння волосся, автоімунне за походженням (алопеція ареата)
Рідкісні (можуть впливати до 1 людини з 1000)
- Надмірна активація білих кров'яних тілець, пов'язана з запаленням (гемофагоцитарний лімфогістіоцитоз)
- Автоімунний розлад згортання крові (пурпура тромботична тромбоцитопенічна, ПТТ)
Частота невідома (не може бути оцінена на основі наявних даних)
- Лістеріоз/менінгіт, викликаний лістерією
- Кровотеча в легенях
- Інфаркт міокарда
- Інсульт (удар)
- Розриви в позвоночних чи каротидних артеріях (кровоносних судинах, які постачають кров до мозку)
- Інфекція, викликана вірусом Епштейна-Барра
- Запалення, яке впливає на кілька органів, захворювання Стілла у дорослих (ДСУ)
Покажіть Карточку пацієнта та цей листок будь-якому лікареві, який пов'язаний з вашим лікуванням, та не тільки неврологу.
Ви також знайдете цю інформацію в Карточці пацієнта та Посібництві для пацієнта, яке ваш лікар дав вам.
Звіт про побічні ефекти
Якщо ви trảiли будь-який побічний ефект, зверніться до свого лікаря, навіть якщо це можливі побічні ефекти, які не вказані в цьому листку. Ви також можете повідомити про них безпосередньо через національну систему повідомлення, включену до Додатку V. Звітуючи про побічні ефекти, ви можете допомогти забезпечити більше інформації про безпеку цього лікарського засобу.
5. Зберігання ЛЕМТРАДИ
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовувати цей лікарський засіб після закінчення терміну придатності, вказаного на коробці та етикетці флакона після CAD. Термін придатності - останній день місяця, який вказано.
Зберігати в холодильнику (між 2 °C та 8 °C).
Не заморожувати.
Зберігати в оригінальній упаковці, щоб захистити від світла.
Рекомендується використовувати продукт негайно після розведення через можливий ризик мікробної контамінації. Якщо продукт не використовується негайно, умови та терміни зберігання, які використовуються до його використання, є відповідальністю користувача та не повинні перевищувати 8 годин при температурі 2 °C до 8 °C, захищеного від світла.
6. Зміст упаковки та додаткова інформація
Склад ЛЕМТРАДА
Активний інгредієнт- алемтузумаб.
Кожна флакон містить 12 мг алемтузумабу в 1,2 мл.
Інші компоненти:
- дигідрат дифосфату натрію (Е339)
- дигідрат едетату натрію
- хлорид потасію (Е508)
- дигідрогенфосфат потасію (Е340)
- полісорбат 80 (Е433)
- хлорид натрію
- вода для ін'єкцій
ВиглядЛЕМТРАДАта вміст упаковки
ЛЕМТРАДА - це концентрат для розчину для інфузії (стерильний концентрат) прозорий або легкий жовтий колір, який міститься у скляному флаконі з пробкою.
У кожній коробці міститься 1 флакон.
Власник дозволу на торгівлю
Sanofi Belgium
Leonardo Da Vincilaan 19
B-1831 Diegem
Бельгія
Виробник
Genzyme Ireland Limited
IDA Industrial Park
Old Kilmeaden Road
Waterford
Ірландія
Для отримання додаткової інформації щодо цього лікарського засобу зверніться до місцевого представника власника дозволу на торгівлю:
Бельгія/Бельгія/Бельгія/Люксембург/Люксембург Sanofi Belgium Тел./Телефон: + 32 2 710 54 00 | Литва Swixx Biopharma UAB Тел.: +370 5 236 91 40 |
| Угорщина SANOFI-AVENTIS Zrt Тел.: +36 1 505 0050 |
Чехія sanofi-aventis, s.r.o. Тел.: +420 233086 111 | Мальта Sanofi S..l. Тел.: +39 02 39394275 |
Данія Sanofi A/S Телефон: +45 45 16 70 00 | Нідерланди Sanofi B.V. Тел.: +31 20 245 4000 |
Німеччина Sanofi Belgium Тел.: +49 (0) 6102 3674 451 | Норвегія sanofi-aventis Norge AS Телефон: + 47 67 10 71 00 |
Естонія Swixx Biopharma OÜ Тел.: +372 640 10 30 | Австрія sanofi-aventis GmbH Тел.: + 43 1 80 185 - 0 |
Греція Sanofi-Aventis Μονοπρ?σωπη AEBE Телефон: +30 210 900 16 00 | Польща Sanofi Sp. z o.o. Тел.: +48 22 280 00 00 |
Іспанія sanofi-aventis, S.A. Тел.: +34 93 485 94 00 | Португалія Sanofi – Produtos Farmacêuticos, Lda. Тел.: +351 21 35 89 400 |
Франція Sanofi Winthrop Industrie Телефон: 0 800 222 555 Дзвінок з-за кордону: +33 1 57 63 23 23 | Румунія Sanofi Romania SRL Тел.: +40 (0) 21 317 31 36 |
Хорватія Swixx Biopharma d.o.o. Тел.: +385 1 2078 500 | Словенія Swixx Biopharma d.o.o. Тел.: +386 1 235 51 00 |
Ісландія Vistor hf. Телефон: +354 535 7000 | Словаччина Swixx Biopharma Тел.: +421 2 208 33 600 |
Ірландія sanofi-aventis Ireland Ltd. T/A SANOFI Тел.: +44353 (0) 403 56 00 | Фінляндія Sanofi Oy Телефон/Тел.: + 358 201 200 300 |
Італія Sanofi S.r.l. Тел.: 800536389 | Швеція Sanofi AB Тел.: +46 (0)8 634 50 00 |
Кіпр C.A. Papaellinas Ltd. Телефон: +357 22 741741 | Велика Британія (Північна Ірландія) sanofi-aventis Ireland Ltd. T/A SANOFI Тел.: +44 (0) 800 035 2525 |
Латвія Swixx Biopharma SIA Тел.: +371 6 616 47 50CP |
Дата останнього перегляду цієї інформації: .
Інші джерела інформації
Для допомоги пацієнтам у формуванні інформації про можливі побічні ефекти та інструкції щодо дій у разі виникнення певних побічних ефектів доступні наступні матеріали з мінімізації ризиків:
1 Карта пацієнта: для того, щоб пацієнт міг показати її іншим медичним працівникам, щоб попередити їх про використання ЛЕМТРАДА пацієнтом.
2 Посібник для пацієнта: для отримання більшої інформації про аутоімунні реакції, інфекції та інші типи інформації.
Детальна інформація про цей лікарський засіб доступна на сайті Європейського агентства з лікарських засобів: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
Ця інформація призначена лише для медичних працівників:
Інформація про мінімізацію ризиків – аутоімунні захворювання
- Надзвичайно важливо, щоб пацієнт зрозумів зобов'язання проходити періодичні тести (під час 4 років після останньої інфузії), навіть якщо немає симптомів і захворювання добре контролюється.
- Разом з пацієнтом потрібно спланувати та керувати періодичними перевірками.
- Пацієнти, які не виконують інструкцій, можуть потребувати додаткової консультації для підкреслення ризиків пропуску періодичних тестів.
- Потрібно контролювати результати тестів і бути уважним до будь-яких симптомів побічної реакції.
- Перегляньте інструкцію та посібник для пацієнта ЛЕМТРАДА з пацієнтом. Напомніть пацієнту, що він повинен бути уважним до будь-яких симптомів, пов'язаних з аутоімунними захворюваннями, і що він повинен звернутися за медичною допомогою у разі сумнівів.
Також доступні освітні матеріали для медичних працівників:
- Посібник ЛЕМТРАДА для медичних працівників
- Модуль навчання ЛЕМТРАДА
- Перелік для лікаря-ordonatora ЛЕМТРАДА
Прочитайте інструкцію (доступна на сайті Європейського агентства з лікарських засобів, згаданому вище) для отримання більшої інформації.
Інформація про підготовку адміністрації ЛЕМТРАДАта нагляд за пацієнтом
- Пацієнти повинні приймати кортикостероїди негайно перед інфузією ЛЕМТРАДА протягом перших 3 днів будь-якого циклу лікування. Також можна розглянути попереднє лікування антигістамінами і/або антипіретиками перед адміністрацією ЛЕМТРАДА.
- Під час лікування та до 1 місяця після нього потрібно призначити пацієнтам антигерпетичний препарат перорально. У клінічних дослідженнях пацієнтам призначали 200 мг ацикловіру двічі на день або еквівалент.
- Базові тести та скринінг описані в розділі 4 інструкції.
- Вміст флакона потрібно візуально перевірити перед кожною адміністрацією, щоб виключити наявність частинок або забарвлення. Якщо концентрат містить частинки або має забарвлення, його не слід використовувати. НЕ ВИТРЯСАЙТЕ ФЛАКОНИ ПЕРЕД ВИКОРИСТАННЯМ.
- Використовуйте асептичні техніки для видалення 1,2 мл ЛЕМТРАДА з флакона та введення в 100 мл розчину для інфузії хлориду натрію (9 мг/мл (0,9%)) або розчину для інфузії глюкози (5%). Мішку потрібно повернути для змішування розчину. Потрібно бути обережним, щоб забезпечити стерильність підготовленого розчину.
- Введіть розчин для інфузії ЛЕМТРАДА внутрішньовенно протягом періоду близько 4 годин.
- Не слід додавати інші лікарські засоби до розчину для інфузії ЛЕМТРАДА чи вводити їх одночасно через ту саму внутрішньовенну лінію.
- Рекомендується використовувати продукт негайно після розведення через можливий ризик мікробної контамінації. Якщо продукт не використовується негайно, умови та терміни зберігання, використані до його використання, є відповідальністю користувача і не повинні перевищувати 8 годин при температурі 2°C до 8°C, захищеному від світла.
- Потрібно слідувати належним процедурам обробки та утилізації. Утилізація будь-яких залишків або матеріалів відходів повинна здійснюватися згідно з місцевими правилами.
Після кожної інфузії потрібно спостерігати за пацієнтом протягом 2 годин для виявлення можливих реакцій, пов'язаних з інфузією. Можна розпочати симптоматичне лікування, якщо це необхідно - див. інструкцію. Продовжуйте проводити аналітичні тести пацієнту кожний місяць для виявлення аутоімунних захворювань до 4 років після останньої інфузії. Див. посібник ЛЕМТРАДА для медичних працівників для отримання більшої інформації або прочитайте інструкцію, доступну на сайті Європейського агентства з лікарських засобів, згаданому вище.
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ЛЕМТРАДА 12 мг концентрат для приготування розчину для інфузійФорма випуску: РОЗЧИН ДЛЯ ІНФУЗІЙ, ЩО ВВОДИТЬСЯ ІН'ЄКЦІЙНО, 120 мг (80 мг/кг) белімумабДіючі речовини: belimumabВиробник: Glaxosmithkline (Ireland) LimitedПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 200 мгДіючі речовини: belimumabВиробник: Glaxosmithkline (Ireland) LimitedПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІНФУЗІЙ, ЩО ВВОДИТЬСЯ ІН'ЄКЦІЙНО, 400 мг (80 мг/кг) белімумабДіючі речовини: belimumabВиробник: Glaxosmithkline (Ireland) LimitedПотрібен рецепт
Лікарі онлайн щодо ЛЕМТРАДА 12 мг концентрат для приготування розчину для інфузій
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ЛЕМТРАДА 12 мг концентрат для приготування розчину для інфузій – за рішенням лікаря та згідно з місцевими правилами.






