INBRIJA 33 MG POLVO PARA INHALACION CAPSULAS DURAS

Cómo usar INBRIJA 33 MG POLVO PARA INHALACION CAPSULAS DURAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Inbrija 33 mg polvo para inhalación, cápsulas duras
levodopa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Inbrija y para qué se utiliza
- Qué necesita saber antes de empezar a usar Inbrija
- Cómo usar Inbrija
- Posibles efectos adversos
- Conservación de Inbrija
- Contenido del envase e información adicional
1. Qué es Inbrija y para qué se utiliza
La sustancia activa en Inbrija es levodopa. Inbrija es un medicamento administrado mediante inhalación para tratar el empeoramiento de sus síntomas durante los periodos OFF de la enfermedad de Parkinson.
La enfermedad de Parkinson afecta al movimiento y se trata con un medicamento que usted toma regularmente. Durante los periodos OFF, su medicina habitual no controla suficientemente bien la enfermedad y es probable que el movimiento sea más difícil.
Debe continuar tomando su medicamento principal para la enfermedad de Parkinson y usar Inbrija para controlar el empeoramiento de los síntomas (como la incapacidad para moverse) durante los periodos OFF.
2. Qué necesita saber antes de empezar a usar Inbrija
No use Inbrija
- si es alérgico a la levodopao a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene visión borrosa, ojos enrojecidos, dolor intenso de ojos y de cabeza, halos alrededor de las luces, el tamaño de las pupilas de sus ojos es más grande de lo habitual y se siente mareado. Si tiene cualquiera de estos síntomas, es posible que tenga glaucoma de ángulo estrecho,un trastorno que aparece de manera repentina: dejede tomar Inbrija y acuda urgentemente al médico.
- si tiene un tumor raro de la glándula suprarrenalllamado feocromocitoma.
- si está tomando determinados medicamentos antidepresivos llamados inhibidores de la MAO no selectivos(p. ej. isocarboxazida y fenelzina). Debe dejar de tomar estos medicamentos al menos 14 días antes de comenzar el tratamiento con Inbrija. Consulte también «Otros medicamentos e Inbrija».
- si en el pasado ha sufrido un síndrome neuroléptico maligno,una reacción potencialmente mortal frente a determinados medicamentos empleados para el tratamiento de trastornos mentales graves, o si ha padecido rabdomiólisis no traumática, un trastorno muscular raro en el que los músculos se destruyen rápidamente.
Advertencias y precauciones
Acuda inmediatamente al médico si tienetemblores, agitación, confusión, fiebre, pulso rápido o mareo o desvanecimiento después de ponerse de pie, o si nota que sus músculos se ponen muy rígidos o tiene espasmos musculares violentos. Pueden ser síntomas de «hiperpirexia por suspensión del medicamento». Para más información consulte la sección 4.
Consulte a su médico o farmacéuticoantes de empezar a usar Inbrija si tiene, ha tenido o desarrolla:
- asma, dificultades para respirar como enfermedad pulmonar obstructiva crónica (EPOC) u otras enfermedades pulmonares o problemas respiratorios prolongados;
- cualquier forma de trastorno mental grave, como psicosis;
- un infarto de miocardio o problemas relacionados con el latido cardíaco. Su médico le monitorizará atentamente durante el inicio del tratamiento;
- una úlcera en el estómago o el intestino;
- un trastorno ocular llamado glaucoma, porque en este caso se debe controlar la presión ocular;
- problemas graves en sus riñones;
- problemas graves en su hígado.
Si no está seguro de presentar alguno de los síntomas mencionados, consulte a su médico o farmacéutico antes de usar Inbrija.
Consulte a su médico o farmacéuticosi desarrolla alguno de los síntomas siguientes mientras esté usando Inbrija:
- ataques repentinos de sueñoo en ocasiones se siente con mucho sueño;
- cambios o un empeoramiento en su estado mentalque pueden ser de carácter grave, como un comportamiento psicótico y suicida;
- alucinaciones,además de confusión, dificultades para dormir, y soñar mucho. Alteración de la actividad mental, como ansiedad, depresión, estar agitado, estar paranoico, con ideas delirantes o desorientación, comportamiento agresivo y delirio;
- empeoramiento de cualquiera de los síntomas respiratorioso si tiene una infección respiratoria;
- impulsos o ansiasde comportarse de maneras poco habituales o que no es capaz de resistirse al impulso, el deseo o la tentación de llevar a cabo determinadas actividades que podrían resultar dañinas para usted o para otras personas. Estas conductas se denominan trastornos del control de impulsos y pueden incluir adicción al juego, comer o gastar en exceso y un deseo sexual anormalmente elevado o un aumento de los pensamientos o sentimientos sexuales. Su médico podría tener que revisar sus tratamientos.
- aparición o intensificación del movimiento anormal del cuerpo(disquinesia);
- sensación de mareo al levantarse(tensión baja);
- melanoma(un tipo de cáncer de piel) o crecimientos o marcas cutáneas sospechosas.
Si va a someterse a una intervención quirúrgica informe a su médico de que está usando Inbrija.
Pruebas
Durante el tratamiento prolongado con sus medicamentos es posible que deba hacerse pruebas del corazón, el hígado, el riñón y hemogramas. Si va a someterse a análisis de sangre o de orina informe a su médico o enfermero de que está tomando Inbrija. Esto se debe a que el medicamento puede influir sobre los resultados de algunas pruebas.
Niños y adolescentes
El uso de Inbrija no está recomendado en pacientes menores de 18 años de edad.
Otros medicamentos e Inbrija
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto se debe a que algunos medicamentos pueden influir sobre el modo en que actúa Inbrija.
No useInbrija si en los últimos 14 días ha tomado fármacos llamados inhibidores de la MAO no selectivos para el tratamiento de la depresión. Estos medicamentos incluyen la isocarboxazida y la fenelzina. En caso de que los esté tomando, hable con su médico o farmacéutico antes de iniciar el tratamiento con Inbrija.
Informe a su médico o farmacéuticosi está tomando:
- medicamentos para su enfermedad de Parkinson llamados inhibidores de la MAO selectivos, como la rasagilina, selegilina y safinamida, inhibidores de la COMT como la entacapona, tolcapona y opicapona, o anticolinérgicos como orfenadrina y trihexifenidilo;
- medicamentos para enfermedades mentales incluida la esquizofrenia, como benperidol, haloperidol, risperidona clorpromazina, flufenazina decanoato, fenotiazina, butirofenona o trifluoperazina;
- metoclopramida para tratar las náuseas;
- isoniazida, un antibiótico para tratar la tuberculosis;
- medicamentos para la hipertensión, porque puede ser necesario ajustar la dosis;
- medicamentos para la depresión llamados antidepresivos tricíclicos, como clomipramina, desipramina o doxepina;
- amantadina para tratar la gripe o su enfermedad de Parkinson.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se recomienda el tratamiento con Inbrija durante el embarazo ni en mujeres en edad fértil que no estén utilizando métodos anticonceptivos.
Las mujeres no deben dar el pecho durante el tratamiento con Inbrija.
Conducción y uso de máquinas
Inbrija puede provocar exceso de somnolencia, mareoy ataques repentinos de sueño. Si experimenta estos síntomas noconduzca ni utilice herramientas ni máquinas. Antes de volver a conducir o a usar máquinas debe estar seguro de que ya no tiene ataques repentinos de sueño, mareo ni somnolencia. De lo contrario podría ponerse a sí mismo o a otros en riesgo de sufrir lesiones graves o muerte.
3. Cómo usar Inbrija
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Antes de empezar a tomar Inbrija debe estar tomando regularmente un tratamiento para la enfermedad de Parkinson que combine un llamado inhibidor de la dopa-descarboxilasa con levodopa.
La dosis recomendada de Inbrija son 2 cápsulaspara el tratamiento de un periodo OFF. No use más de 2 cápsulas para cada periodo OFF. Puede usar 2 cápsulas hasta cinco veces al día.
La dosis máxima de Inbrija es 10 cápsulas al día.
Información importante antes de usar Inbrija:
- Las cápsulas duras Inbrija no se deben tragar.
- Este medicamento se usa únicamente por inhalación.
- Saque las cápsulas del blíster justo antes de utilizarlas.
- Se deben inhalar dos cápsulas del medicamento para recibir la dosis completa.
- El medicamento solo debe usarse con el inhalador Inbrija.
- Cuando abra una caja nueva use siempre el inhalador nuevo.
- Su médico o farmacéutico le enseñarán cómo usar correctamente el medicamento.
Consulte en las «Instrucciones de uso»al final de este prospecto cómo usar su medicamento con el inhalador incluido.
Si usa más Inbrija del que debe
Si usa más Inbrija del que debe (o alguien toma Inbrija de manera accidental), acuda inmediatamente al médico.Es posible que se sienta confundido o en estado de agitación, y su frecuencia cardíaca puede ser más lenta o más rápida de lo normal.
Si olvidó usar Inbrija
Use Inbrija solo durante un periodo OFF. Si el periodo OFF ha pasado, no use Inbrija hasta que se produzca el siguiente periodo OFF.
Si interrumpe el tratamiento con Inbrija
No deje de usar Inbrija sin verificarlo con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Acuda inmediatamente al médico si tieneun edema alérgico con síntomas como urticaria (ronchas), picor, exantema, hinchazón de su cara, labios, lengua o garganta. Esto puede provocar dificultades para respirar o tragar.
Acuda urgentemente al médico sisus músculos se ponen muy rígidos o tiene espasmos musculares violentos, temblores, agitación, confusión, fiebre, pulso rápido o fuertes fluctuaciones en su tensión arterial. Pueden ser síntomas de un síndrome neuroléptico maligno (SNM, una reacción rara grave a los medicamentos usados para tratar enfermedades del sistema nervioso central) o rabdomiólisis (una enfermedad muscular grave rara).
Acuda urgentemente al médico si tienehemorragia en el estómago o intestinos, las puede detectar como sangre en sus heces o heces más oscuras.
El uso de este medicamento puede provocar los siguientes efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- tos
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- aparición o intensificación de movimientos anormales del cuerpo (disquinesia);
- infecciones de nariz, senos paranasales, garganta o pulmones;
- alteración del color de la mucosidad;
- alteración del color de la mucosidad nasal (es decir, no es transparente);
- irritación o picor de garganta;
- sensación de malestar (náuseas); vómitos;
- tener tendencia a sufrir caídas.
Otros efectos adversos de frecuencia desconocida que puede experimentar son:
- sensación de asfixia asociada al impacto del polvo del medicamento en la parte posterior de la garganta, inmediatamente después del uso;
- cáncer de piel;
- deficiencia de glóbulos rojos, que provoca palidez y sensación de cansancio; tener más tendencia a contraer infecciones por un déficit de leucocitos; falta de plaquetas que puede provocar moratones y tendencia a sangrar;
- pérdida del apetito;
- confusión; alucinaciones; depresión; ansiedad; pesadillas; insomnio; alteración de la actividad mental y de las percepciones, pérdida del contacto con la realidad; sensación de estar agitado; comportamiento suicida; estar desorientado; sentimiento exagerado de felicidad; aumento de la líbido; bruxismo; sentimiento paranoide o delirante;
- trastorno del movimiento donde los músculos de la persona se contraen de manera incontrolable; cambios repentinos, en ocasiones impredecibles, en los síntomas por la reaparición de los síntomas de la enfermedad de Parkinson; somnolencia; mareo; empeoramiento de la enfermedad de Parkinson; hormigueo; dolor de cabeza; temblores; convulsión; aparición repentina de sueño; síndrome de las piernas inquietas; ataxia (trastorno que afecta a la coordinación, el equilibrio y el habla); sentido del gusto alterado; trastornos mentales que afectan al aprendizaje, la memoria, la percepción y la resolución de problemas; síndrome de Horner (una enfermedad ocular); demencia;
- visión borrosa; visión doble; agrandamiento de las pupilas; ojos en blanco durante un tiempo prolongado; cierre fuerte e involuntario de los párpados;
- problemas cardíacos, un latido marcadamente rápido, fuerte o irregular;
- tensión baja poco después de levantarse; tensión alta; desvanecimiento; coágulo en una vena; sofocos;
- disnea; dificultad para respirar; dificultad en el habla; hipo;
- dolor estomacal; estreñimiento; diarrea; boca seca; hemorragia estomacal o intestinal; úlcera de estómago; dificultad en la deglución; indigestión; sensación de quemazón en la boca; flatulencia; alteración del color de la saliva; más saliva de lo normal;
- cara, labios, lengua, extremidades y genitales inflamados; exceso de sudoración; exantema; picor intenso de la piel; una enfermedad llamada púrpura de Schoenlein Henoch, cuyos síntomas incluyen erupción cutánea con manchas de color púrpura; reacción alérgica que provoca un exantema de ronchas redondas y rojas en la piel que pican de manera intensa; pérdida de cabello; alteración del color del sudor;
- espasmos musculares; trismo;
- dificultad para vaciar la vejiga; alteración del color de la orina; pérdida del control de la vejiga;
- erección dolorosa y anormalmente duradera;
- hinchazón de la parte inferior de las piernas o las manos; sensación de debilidad y falta de energía; sensación de cansancio; pérdida de energía; dificultad para andar; dolor en el pecho;
- resultados de los analisis de sangre ánomalos; pérdida de peso; aumento de peso.
También puede experimentar los siguientes efectos adversos:
- incapacidad de resistirse al impulso de realizar una acción que podría resultar dañina, como por ejemplo:
- fuerte impulso de jugar en exceso a pesar de las graves consecuencias personales o familiares;
- alteración o aumento del deseo y la conducta sexual que puedan resultar de especial preocupación para usted o para otras personas, por ejemplo un aumento del impulso sexual;
- comprar o gastar de forma excesiva e incontrolable;
- darse atracones de comida (comer grandes cantidades de alimentos en un corto periodo de tiempo) o comer de forma compulsiva (comer más de lo normal y más de lo necesario para saciar el hambre).
Informe a su médico si experimenta alguna de estas conductas; este analizará con usted formas de tratar o reducir los síntomas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Inbrija
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en los blísteres y la caja después de EXP o CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar por debajo de 25 °C. Conservar en el embalaje original para protegerlo de la luz y la humedad y sacarlo justo antes de usar.
No usar las cápsulas si están aplastadas, dañadas o húmedas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Inbrija
- El principio activo es levodopa. Cada cápsula dura contiene 42 mg de levodopa. La dosis que sale por la boquilla del inhalador (dosis administrada) contiene 33 mg de levodopa.
- Los demás componentes del polvo y la cápsula son palmitato de colfoscerilo (DPPC), cloruro sódico, hipromelosa, dióxido de titanio (E 171), carragenina, cloruro de potasio, cera de carnauba, almidón de maíz, goma laca, óxido de hierro negro (E 172), propilenglicol e hidróxido de potasio.
Aspecto del producto y contenido del envase
Inbrija polvo para inhalación, cápsulas duras, consiste en un polvo blanco para la inhalación metido en cápsulas duras opacas con «A42» impreso en negro sobre la tapa de la cápsula y dos franjas negras impresas sobre el cuerpo de la cápsula.
En esta caja encontrará un inhalador junto con blísteres pelables de 4 cápsulas duras cada uno.
Los tamaños de envase son
- una caja con 16 cápsulas duras (tira de 4 blísteres) y un inhalador
- una caja con 32 cápsulas duras (tira de 8 blísteres) y un inhalador
- una caja con 60 cápsulas duras (tira de 15 blísteres) y un inhalador
- una caja con 92 cápsulas duras (tira de 23 blísteres) y un inhalador
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Acorda Therapeutics Ireland Limited
10 Earlsfort Terrace
Dublin 2, D02 T380
Irlanda
Tel: +353 (0)1 231 4609
Fabricante
ADOH B.V.
Godfried Bomansstraat 31
6543 JA Nijmegen
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Acorda Therapeutics Ireland Limited Tél/Tel: +353 (0)1 231 4609 | Lietuva Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
???????? Acorda Therapeutics Ireland Limited Te?.: +353 (0)1 231 4609 | Luxembourg/Luxemburg Acorda Therapeutics Ireland Limited Tél/Tel: +353 (0)1 231 4609 |
Ceská republika Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Magyarország Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Danmark Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Sverige Tlf.: +46 8 368000 | Malta Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Deutschland Merz Therapeutics GmbH Eckenheimer Landstraße 100 60318 Frankfurt/Main Tel: +49 (0) 69 1503 0 | Nederland Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Eesti Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 | Norge Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Sverige Tlf: +46 8 368000 |
Ελλ?δα Acorda Therapeutics Ireland Limited Τηλ: +353 (0)1 231 4609 | Österreich Merz Pharma Austria GmbH Guglgasse 17 1110 Wien Tel: +43 (0) 1 865 88 95 |
España Esteve Pharmaceuticals S.A. Passeig de la Zona Franca, 109, planta 4 08038 Barcelona España Tel: +34 93 446 60 00 | Polska Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
France Merz Pharma France Tour EQHO 2, Avenue Gambetta 92400 Courbevoie Tél: +33 1 47 29 16 77 | Portugal Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Hrvatska Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 Ireland Merz Pharma UK Ltd. Suite B, Breakspear Park, Breakspear Way Hemel Hempstead Hertfordshire HP2 4TZ United Kingdom Tel: +44 (0) 208 236 0000 | România Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 Slovenija Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Ísland Acorda Therapeutics Ireland Limited Sími: +353 (0)1 231 4609 | Slovenská republika Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Italia Merz Pharma Italia Srl Via Fabio Filzi 25 A 20124 Milan Tel: +39 02 66 989 111 | Suomi/Finland Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Ruotsi Puh/Tel: +46 8 368000 |
Κ?προς Acorda Therapeutics Ireland Limited Τηλ: +353 (0)1 231 4609 | Sverige Merz Therapeutics Nordics AB Gustav III S Boulevard 32 Regus Solna 169 73 Tel: +46 8 368000 |
Latvija Acorda Therapeutics Ireland Limited Tel: +353 (0)1 231 4609 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
----------------------------------------------------------------------------------------------------------------
Instrucciones de uso:
Lea estas instrucciones antes de empezar a usar Inbrija. | ||
Resumen
| ||
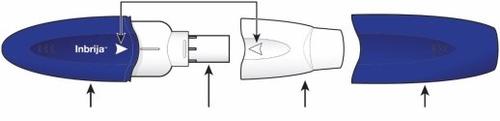
Componentes de su inhalador Inbrija Flechas para la alineación
Mango azul Cámara de la cápsula Boquilla blanca Capuchón azul | ||
Cápsulas | ||
Cada caja contiene blísteres de 4 cápsulas cada uno.
| Prepare y use 2 cápsulas en total. Use cada vez una cápsula.
| Dosis completa = 2 cápsulas.
|
Prepare su dosis | ||
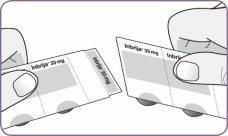
| Busque una superficie limpia y seca. Asegúrese de que tiene las manos limpias y secas. Coja el inhalador y la tira de las cápsulas. Abra el envase de 2 cápsulas. Una dosis completa son 2 cápsulas. | |
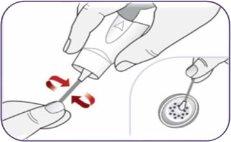
Paso 2: Quitar el capuchón azul del inhalador
| Tire del capuchón para quitarlo. Deje el capuchón a un lado. Lo necesitará después para guardar el inhalador. | |
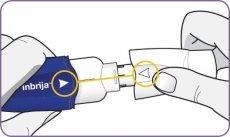
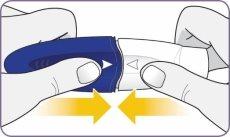
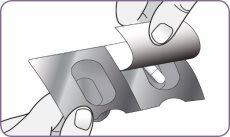
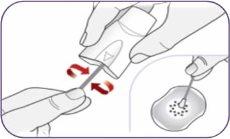
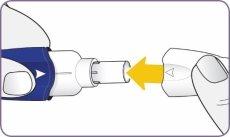
| Gire la boquilla y tire de ella para quitarla del mango. Deje la boquilla y el inhalador sobre una superficie limpia y seca. | |
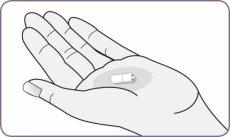
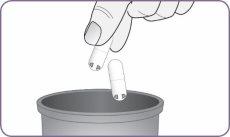
| Desprenda con cuidado la lámina del envase y saque 1 cápsula. Saque solo 1 cápsula cada vez y justo antes de usarla. No use las cápsulas si están aplastadas, dañadas o húmedas. En este caso, deséchela y coja una nueva. | |
| Mantenga el inhalador en vertical cogiéndolo por el mango. Deje caer la primera cápsula en el interior del orificio de la cámara de la cápsula. No cargue 2 cápsulas al mismo tiempo. |
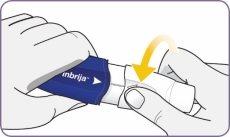
Paso 6: Montar la boquilla blanca | |
Alinear las flechas en la boquilla y el mango
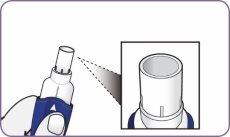
| Alinee las flechas blancas en el mango y la boquilla. |
Presionar la boquilla solo una vez
| Presione con firmeza la boquilla contra el mango hasta que escuche un clic. Con esta maniobra se perfora la cápsula. No presione el mango y la boquilla más de una vez. |
Soltar la boquilla
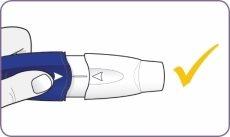
| Suelte la boquilla. La boquilla regresa a su posición y se queda montada. |
Ahora, el inhalador está listo para usar. No presione el mango y la boquilla más de una vez. De lo contrario podría dañar la cápsula y no tomar la dosis completa. Si esto sucede, empiece de nuevo por el paso 4 usando una cápsula nueva. Compruebe que la boquilla esté firmemente sujeta y que no se cae antes de pasar al paso 7. | |
Tome su dosis | |
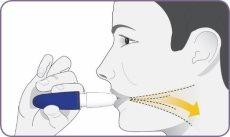
Paso 7: Alejar el inhalador y espirar
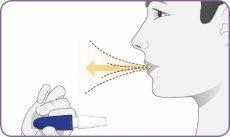
| Póngase en posición erguida o sentada con la cabeza y el pecho rectos. Mantenga el inhalador a la altura de la boca pero alejado de ella. Espire por completo. No espire en el interior de la boquilla. |
Paso 8: Inspirar profundamente para inhalar el polvo
| Mantenga el inhalador a la altura de la boca, y cierre firmemente los labios alrededor de la boquilla. Inspire profunda y ampliamente hasta que sienta los pulmones llenos. Es normal que tarde unos segundos. Mientras inspira escuchará y notará cómo la cápsula «gira». Este giro indica que el inhalador está funcionando y que usted está recibiendo su medicamento. Si tose o interrumpe la dosis empiece de nuevo desde el paso 7 usando la misma cápsula. Importante: si no ha oído o sentido el giro de la cápsula durante la inhalación, es posible que deba inspirar de un modo más profundo y prolongado, o que tenga que limpiar la boquilla. (No enjuague la boquilla ni moje el inhalador). Consulte el paso 13: Limpieza de la boquilla. Empiece de nuevo desde el paso 7 usando la misma cápsula. |
Paso 9: Aguantar la respiración durante 5 segundos y espirar
| Retire el inhalador de la boca y mantenga a respiración durante 5 segundos. Después, espire. |
Paso 10:Sacar la cápsula del envase | |
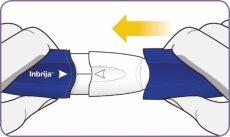
Girar y quitar la boquilla
| Gire y quite la boquilla. |
Sacar la cápsula usada
| Saque la cápsula usada. |
Paso 11:Dosis con la 2ª cápsula
| Repita los pasos de 4 a 10 con una segunda cápsula para acabar la dosis completa. Debe inhalar el contenido de la segunda cápsula durante los 10 minutos posteriores a la primera. |
Eliminación y almacenamiento | |
Paso 12:Eliminación de las cápsulas usadas
| Elimine las cápsulas usadas según las disposiciones locales. |
Paso 13: Limpieza de la boquilla Es normal que quede un poco de polvo en o sobre el inhalador. Para evitar su acumulación, elimine el polvo de los orificios de la boquilla cuando sea necesario haciendo movimientos circulares con un bastoncillo de algodón seco nuevo. | |
Limpieza de los orificios de la parte superior de la boquilla
| Limpie los orificios de la parte superior de la boquilla. |
Limpieza de los orificios de la parte inferior de la boquilla
| Limpie los orificios de la parte inferior de la boquilla. |
También puede usar un pañuelo de papel seco para frotar el exterior de la boquilla cuando sea necesario. No limpie ninguna otra parte del inhalador. No aclare la boquilla ni moje el inhalador. | |
Paso 14: Almacenar el inhalador | |
Comprobar que no hay ninguna cápsula en el inhalador
| Compruebe que no hay ninguna cápsula en el inhalador antes de guardarlo. |
Montar la boquilla
| Monte la boquilla en el mango presionándola hasta que escuche un clic. |
Colocar el capuchón
| Coloque el capuchón sobre la boquilla. |
Listo para guardar
| Ahora el inhalador está listo para guardar. |
Limpieza de inhalador
|
- País de registro
- Precio medio en farmacia354.55 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a INBRIJA 33 MG POLVO PARA INHALACION CAPSULAS DURASForma farmacéutica: COMPRIMIDO, 10 mg/100 mgPrincipio activo: levodopa and decarboxylase inhibitorFabricante: Fairmed Healthcare GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 12,5 mg/50 mgPrincipio activo: levodopa and decarboxylase inhibitorFabricante: Fairmed Healthcare GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 25 mg/100 mgPrincipio activo: levodopa and decarboxylase inhibitorFabricante: Fairmed Healthcare GmbhRequiere receta
Médicos online para INBRIJA 33 MG POLVO PARA INHALACION CAPSULAS DURAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de INBRIJA 33 MG POLVO PARA INHALACION CAPSULAS DURAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







 Paso 1: Preparativos
Paso 1: Preparativos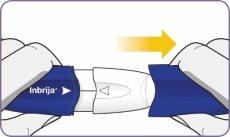
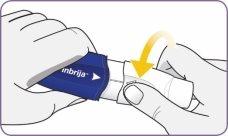 Paso 3: Girar y quitar la boquilla blanca
Paso 3: Girar y quitar la boquilla blanca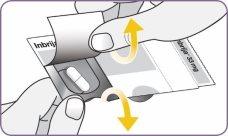 Paso 4: Sacar 1 cápsula del envase
Paso 4: Sacar 1 cápsula del envase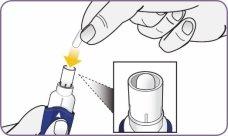 Paso 5: Cargar la cápsula
Paso 5: Cargar la cápsula