HYQVIA 100 MG/ML SOLUCION PARA PERFUSION


Cómo usar HYQVIA 100 MG/ML SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
HyQvia100mg/ml, solución para perfusión
inmunoglobulina humana normal
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es HyQvia y para qué se utiliza
- Qué necesita saber antes de empezar a usar HyQvia
- Cómo usar HyQvia
- Posibles efectos adversos
- Conservación de HyQvia
- Contenido del envase e información adicional
1. Qué es HyQvia y para qué se utiliza
Qué es HyQvia
HyQvia contiene 2 soluciones para perfundir (goteo) bajo la piel (perfusión subcutánea o SC). Se suministra en un envase que contiene:
- un vial de inmunoglobulina humana normal 10% (el principio activo)
- un vial de hialuronidasa humana recombinante (una sustancia que ayuda a que la inmunoglobulina humana normal 10% llegue a la sangre).
La inmunoglobulina humana normal 10% pertenece a una clase de medicamentos denominados “inmunoglobulinas humanas normales”. Las inmunoglobulinas también son anticuerpos y se encuentran en la sangre de las personas sanas. Los anticuerpos forman parte del sistema inmunológico (las defensas naturales del cuerpo) y ayudan al cuerpo a luchar contra las infecciones.
Cómo funciona HyQvia
La hialuronidasa humana recombinante es una proteína que facilita la perfusión (por goteo) de las inmunoglobulinas bajo la piel y su llegada al sistema circulatorio.
El vial de inmunoglobulinas está preparado a partir de la sangre de personas sanas. Las inmunoglobulinas son producidas por el sistema inmunitario del cuerpo humano. Ayudan a su organismo a combatir las infecciones causadas por bacterias y virus o a mantener el equilibrio del sistema inmunitario (lo que se conoce como inmunomodulación). El medicamento actúa del mismo modo que las inmunoglobulinas presentes de forma natural en la sangre.
Para qué se utiliza HyQvia
Terapia de sustitución en adultos y niños (de0a18años de edad)
HyQvia se utiliza en pacientes con un sistema inmunológico débil, que no tienen suficientes anticuerpos en la sangre y son propensos a padecer infecciones, entre los que se incluyen los siguientes grupos:
- pacientes que han nacido con una incapacidad o una capacidad reducida para producir anticuerpos (inmunodeficiencias primarias),
- pacientes que sufren infecciones graves o recurrentes debido a un sistema inmulogógico debilitado resultado de otras afecciones o tratamientos (inmunodeficiencias secundarias).
Las dosis regulares y suficientes de HyQvia pueden aumentar los niveles anormalmente bajos de las inmunoglobulinas en su sangre hasta niveles normales (terapia de sustitución).
Terapia inmunomoduladoraen adultos, niños y adolescentes (de 0 a 18años)
- HyQvia se utiliza en pacientes adultos, niños y adolescentes (de 0 a 18 años) con polineuropatía desmielini zante inflamatoria crónica (PDIC), un tipo de enfermedad autoinmune. La PDIC se caracteriza por una inflamación crónica de los nervios periféricos que provoca debilidad muscular y/o entumecimiento, principalmente en las piernas y los brazos. Se cree que el propio sistema de defensa del organismo ataca los nervios periféricos y provoca lesiones nerviosas e inflamación. Se cree que las inmunoglobulinas presentes en HyQvia ayudan a proteger los nervios de los daños causados por el sistema inmunitario.
2. Qué necesita saber antes de empezar a usar HyQvia
No inyecte ni perfunda HyQvia
- si es alérgico a las inmunoglobulinas, la hialuronidasa, la hialuronidasa recombinante o a alguno de los demás componentes de este medicamento (incluidos en la sección 6, “Contenido del envase e información adicional”)
- si tiene anticuerpos frente a la inmunoglobulina A (IgA) en la sangre. Esto puede ocurrir si tiene déficit de IgA. Como HyQvia contiene trazas de IgA, podría sufrir una reacción alérgica
- en un vaso sanguíneo (de forma intravenosa) ni en un músculo (de forma intramuscular).
Advertencias y precauciones
Consulte a su médico o enfermero/a antes de empezar a usar HyQvia.
- Antes del tratamiento, avise a su médico o profesional sanitario si se le aplica alguna de las circunstancias indicadas a continuación:
- Usted o su hijo pueden ser alérgicos a las inmunoglobulinas y no saberlo. Las reacciones alérgicas, como una bajada repentina de la presión arterial o un shock anafiláctico (una brusca caída de la presión arterial junto con otros síntomas como hinchazón de garganta, dificultad para respirar y erupción cutánea) son raras pero pueden ocurrir aunque no haya tenido problemas anteriormente con tratamientos similares. Tiene mayor riesgo de sufrir reacciones alérgicas si tiene un déficit de IgA con anticuerpos anti‑IgA. Los signos o síntomas de estas reacciones alérgicas raras incluyen:
- sensación de aturdimiento, mareo o pérdida de conocimiento,
- erupción cutánea y picor, hinchazón de la boca o garganta, dificultad para respirar, sibilancias (sonido silbante que se produce al respirar),
- frecuencia cardiaca anormal, dolor en el pecho, tono azulado de labios o dedos de manos y pies,
- visión borrosa
- Si nota cualquiera de estos signos durante la perfusión, informe inmediatamente a su médico o al enfermero. Ellos decidirán si debe reducir la velocidad de perfusión o detenerla completamente.
Su médico o enfermero/a perfundirá hialuronidasa humana recombinante (HY) seguida de inmunoglobulina (Ig) despacio y, con cuidado, le controlará durante la 1ª perfusión para que, en caso de reacción alérgica, se pueda detectar y tratar de inmediato.
- Su médico tendrá especial cuidado si tiene sobrepeso, es un paciente de edad avanzada, tiene diabetes, ha permanecido encamado durante un periodo de tiempo prolongado, tiene la tensión arterial elevada, tiene un volumen bajo de sangre (hipovolemia), tiene problemas en los vasos sanguíneos (enfermedades vasculares), tiene una tendencia aumentada de coagulación de la sangre (trombofilia o episodios trombóticos) o tiene una enfermedad que provoca que la sangre sea más espesa (sangre hiperviscosa). En estas circunstancias, las inmunoglobulinas pueden aumentar el riesgo de ataque al corazón (infarto), ictus, coágulos de sangre en los pulmones (embolia pulmonar) o bloqueo de un vaso sanguíneo de la pierna, aunque en condiciones muy raras.
- Si nota cualquiera de estos signos y síntomas durante la perfusión, incluidos falta de aliento, dolor, hinchazón de una extremidad y dolor en el pecho, informe inmediatamente a su médico o enfermera/o. Ellos decidirán si debe reducir la velocidad de perfusión o detenerla completamente.
Su médico o enfermera/o le supervisará atentamente durante las perfusiones para que, en caso de acontecimientos tromboembólicos, se puedan detectar y tratar de inmediato.
- Recibirá este medicamento en dosis altas en el transcurso de 1 o 2 días, y en caso de pertenecer a los grupos sanguíneos A, B o AB y padecer una enfermedad inflamatoria subyacente. En estas circunstancias, se ha notificado habitualmente que las inmunoglobulinas aumentan el riesgo de destrucción de los glóbulos rojos (hemólisis).
- Se ha notificado inflamación de las membranas que rodean el cerebro y la médula espinal (síndrome de meningitis aséptica) relacionada con el tratamiento con inmunoglobulinas.
- Si nota cualquiera de estos signos y síntomas después de la perfusión, incluidos dolor de cabeza grave, rigidez de nuca, mareo, fiebre, fotofobia, náuseas y vómitos, informe inmediatamente a su médico o al enfermero.
Su médico decidirá si es necesario realizarle más pruebas y si el tratamiento con HyQvia debe continuar.
Velocidad de perfusión
Es muy importante perfundir el medicamento a la velocidad correcta. Su médico o enfermero le aconsejarán la velocidad de perfusión adecuada para cuando se perfunda HyQvia en casa (ver sección 3, “Cómo usar HyQvia”).
Control durante la perfusión
Determinados efectos adversos pueden producirse con más frecuencia si:
- está recibiendo HyQvia por primera vez
- ha recibido otra inmunoglobulina y ha cambiado a HyQvia
- ha pasado mucho tiempo (por ejemplo, más de 2 o 3 intervalos de perfusión) desde la última vez que recibió HyQvia.
- En tales casos, se le controlará con atención durante la primera perfusión y durante la primera hora después de que la perfusión haya acabado.
En los demás casos, se le controlará con atención durante la perfusión y, al menos, 20 minutos después de que haya recibido las primeras perfusiones de HyQvia.
Tratamiento domiciliario
Antes de comenzar el tratamiento domiciliario se le asignará una persona como cuidador. A usted y a su cuidador se les formará para detectar los primeros signos de efectos adversos, especialmente reacciones alérgicas. Este cuidador le ayudará a observar los posibles efectos adversos. Durante la perfusión deberá observar si se producen los primeros signos de efectos adversos (para obtener más detalles, ver sección 4, “Posiblesefectos adversos”).
- Si aprecia cualquier efecto adverso, usted o su cuidador deberán detener la perfusión de inmediato y ponerse en contacto con un médico.
- Si experimenta un efecto adverso grave, usted o su cuidador deberán buscar tratamiento de emergencia de inmediato.
Propagación de infecciones localizadas
No perfundir HyQvia en o alrededor de una zona infectada o hinchada y enrojecida de la piel ya que podría extender la infección.
No se observaron cambios a largo plazo (crónicos) de la piel en los estudios clínicos. Debe comunicarse al médico cualquier inflamación a largo plazo, bultos (nódulos) o inflamación que aparezca en el punto de perfusión y dure más de unos pocos días.
Efectos en los análisis de sangre
HyQvia contiene muchos anticuerpos diferentes, algunos de los cuales pueden interferir con los análisis de sangre (pruebas serológicas).
- Antes de realizarse un análisis de sangre, informe a su médico de su tratamiento con HyQvia.
Información sobre el material de origen de HyQvia
La inmunoglobulina humana normal 10% de HyQvia y la albúmina sérica humana (un componente de la hialuronidasa humana recombinante) se producen a partir del plasma humano (la parte líquida de la sangre). Cuando los medicamentos se elaboran a partir de sangre o plasma humanos, se deben adoptar un número de medidas para prevenir una posible transmisión de infecciones a los pacientes. Entre éstas se incluyen:
- una selección cuidadosa de los donantes de sangre y plasma para garantizar la exclusión de donantes que están en riesgo de ser portadores de enfermedades infecciosas
- el análisis de cada donación y mezcla de plasmas para detectar posibles virus o infecciones.
Los fabricantes de estos productos incluyen además etapas en el proceso de fabricación para eliminar/inactivar virus. A pesar de esto, cuando se usan medicamentos derivados de sangre o plasma humanos, la posibilidad de transmisión de agentes infecciosos no puede excluirse totalmente. Esto también se refiere a virus y agentes infecciosos emergentes o de naturaleza desconocida.
Las medidas adoptadas para la fabricación de HyQvia se consideran eficaces para los virus envueltos como el virus de la inmunodeficiencia humana (VIH), el virus de la hepatitis B (VHB) y el virus de la hepatitis C (VHC) y para los virus no envueltos como el de la hepatitis A y el parvovirus B19.
Las inmunoglobulinas no se han relacionado con las infecciones de hepatitis A o por parvovirus B19, probablemente porque los anticuerpos asociados a estas infecciones (y que se encuentran en HyQvia) ofrecen protección.
- Se recomienda encarecidamente que cada vez que se le administre una dosis de HyQvia, se anoten los siguientes datos en el diario del paciente:
- el nombre del producto,
- la fecha de administración,
- el número de lote del medicamento y
- el volumen inyectado, la velocidad de administración, el número y la ubicación de los puntos de perfusión.
Niños y adolescentes
Terapia de sustitución
Las mismas indicaciones, dosis y frecuencia de perfusión para los adultos se aplican a los niños y adolescentes (0 a 18 años).
Terapia inmunomoduladoraen pacientes con PDIC
No se ha establecido la seguridad y la eficacia de HyQvia en niños y adolescentes (0 a 18 años de edad) con PDIC.
Otros medicamentos y HyQvia
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Vacunas
HyQvia puede reducir el efecto de algunas vacunas, como la del sarampión, rubeola, paperas y varicela (vacunas elaboradas con virus vivos). Por ello, tras recibir HyQvia, puede que tenga que esperar hasta 3 meses antes de recibir determinadas vacunas. Puede que tenga que esperar hasta 1 año después de recibir HyQvia antes de poder recibir la vacuna del sarampión.
- Antes de vacunarse, informe a su médico o enfermero sobre su tratamiento con HyQvia.
Embarazo, lactancia y fertilidad
Los datos sobre los efectos del uso de la hialuronidasa humana recombinante a largo plazo sobre el embarazo, la lactancia y la fertilidad son limitados. HyQvia solo debe utilizarse en mujeres embarazadas o en periodo de lactancia tras considerarlo con el médico.
Conducción y uso de máquinas
Durante el tratamiento con HyQvia, los pacientes podrían experimentar efectos adversos (por ejemplo, mareo o náuseas) que podrían afectar a su capacidad para conducir y utilizar máquinas. Si esto ocurre, debe esperar a que las reacciones desaparezcan.
HyQvia contiene sodio
Este medicamento contiene de 5,0 a 60,5 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada vial de hialuronidasa humana recombinante HyQvia. Esto equivale a entre el 0,25 y el 3 % de la ingesta diaria máxima de sodio recomendada para un adulto.
El componente Ig 10% está básicamente exento de sodio.
3. Cómo usar HyQvia
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
HyQvia tiene que perfundirse bajo la piel (administración subcutánea o SC).
Su médico o enfermero iniciará el tratamiento con HyQvia, pero una vez que haya recibido las primeras perfusiones bajo supervisión médica y usted (y/o su cuidador) estén debidamente formados, podrá utilizar el medicamento en casa. Usted y su médico decidirán si puede utilizar HyQvia en casa. No comience el tratamiento con HyQvia en casa hasta que haya recibido las instrucciones completas.
Dosificación
Terapia de sustitución
Su médico calculará la dosis correcta basándose en el peso corporal, en los tratamientos anteriores que haya recibido y en su respuesta al tratamiento. La dosis de inicio recomendada es una que proporcione de 400 a 800 mg de principio activo por kg de peso corporal al mes. Al principio, recibirá un cuarto de esa dosis en intervalos de 1 semana. Las siguientes perfusiones aumentarán gradualmente a dosis mayores en intervalos de 3 a 4 semanas. A veces, el médico puede recomendar dividir las dosis más grandes y administrarlas en 2 puntos a la vez. El médico también podrá ajustar la dosis dependiendo de su respuesta al tratamiento.
Terapia inmunomoduladora
Su médico calculará la dosis correcta para usted basándose en los tratamientos previos que haya recibido y en su respuesta al tratamiento. Normalmente, el tratamiento comienza 1 o 2 semanas después de la última perfusión de inmunoglobulina administrada subcutáneamente con la dosis semanal equivalente calculada. Su profesional sanitario puede ajustar la dosis y la frecuencia en función de la respuesta al tratamiento.
En caso de que se supere la dosis diaria (> 120 g) o si no puede tolerar el volumen de perfusión de inmunoglobulinas, la dosis se puede dividir y administrar en el transcurso de varios días, dejando entre 48 y 72 horas entre dosis para permitir una correcta absorción; la administración de hialuronidasa también debe dividirse de la forma apropiada.
Inicio del tratamiento
El tratamiento lo iniciará un médico o enfermero con experiencia en el tratamiento de pacientes con un sistema inmunológico débil (inmunodeficiencia) y PDIC en formar a pacientes para el tratamiento domiciliario. Se le observará cuidadosamente durante la perfusión y durante, al menos, 1 hora después para ver si tolera bien el medicamento. Al principio, su médico o enfermero utilizarán una velocidad de perfusión lenta y, gradualmente, la aumentarán durante la primera perfusión y las siguientes. Una vez que el médico o enfermero haya encontrado la dosis y la velocidad de perfusión adecuadas para usted, podrá administrarse el tratamiento domiciliario.
Tratamiento domiciliario
No utilice HyQvia en casa hasta que reciba instrucciones y formación por parte del profesional sanitario.
Se le formará en:
- Técnicas asépticas (sin gérmenes) de perfusión,
- La utilización de una bomba de perfusión o una bomba de perfusión continua (si es necesario),
- El mantenimiento de un diario del paciente y
- Medidas que se deben seguir en caso de reacciones adversas graves.
Deberá seguir minuciosamente las instrucciones de su médico en cuanto a la dosis, la velocidad de perfusión y la planificación a la hora de perfundir HyQvia, para que el tratamiento funcione.
Se recomiendan las siguientes velocidades de perfusión para Ig 10 % por punto de perfusión:
Sujetos<40kg | Sujetos≥40kg | |||
Intervalo/minutos | Primeras 2 perfusiones (ml/hora/punto de perfusión) | 2 a 3 perfusionessiguientes (ml/hora/punto de perfusión) | Primeras 2 perfusiones (ml/hora/punto de perfusión) | 2 a 3 perfusionessiguientes (ml/hora/punto de perfusión) |
10 minutos | 5 | 10 | 10 | 10 |
10 minutos | 10 | 20 | 30 | 30 |
10 minutos | 20 | 40 | 60 | 120 |
10 minutos | 40 | 80 | 120 | 240 |
Resto de la perfusión | 80 | 160 | 240 | 300 |
Las velocidades de perfusión indicadas son para un solo punto de perfusión. En caso de que el paciente necesite 2 o 3 puntos de perfusión, las velocidades de perfusión pueden ajustarse en consecuencia (es decir, duplicarse o triplicarse en función de la velocidad máxima de perfusión de la bomba).
Si se produce una pérdida en el punto de perfusión
Pregunte a su médico, farmacéutico o enfermero si otro tamaño de aguja sería más adecuado para usted. Cualquier cambio en el tamaño de la aguja debe supervisarlo el médico.
Si usa más HyQvia del que debe
Si cree que ha usado más HyQvia del que debe, consulte a su médico lo antes posible.
Si olvidó usar HyQvia
No se administre una dosis doble de HyQvia para compensar las dosis olvidadas. Si cree que ha olvidado una dosis, consulte a su médico lo antes posible.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
En la sección siguiente se ofrecen instrucciones detalladas sobre el uso.
| |
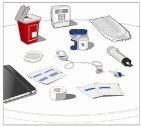
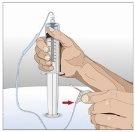
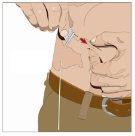
Prepare todos los materialespara la perfusión. Estos incluyen: unidad(es) de vial doble de HyQvia, materiales para la perfusión (aguja subcutánea, envase de la solución (bolsa o jeringa), venda estéril y esparadrapo, tubos de la bomba, dispositivos de transferencia, jeringas, gasa y esparadrapo), contenedor para objetos punzantes, bomba y libro de registro del tratamiento, así como otros materiales que sean necesarios. |
|
| |
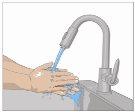
Lávese las manos minuciosamente. Coloque todos los materiales necesarios y ábralos según las indicaciones de su profesional sanitario. |
|
|
|
|
|
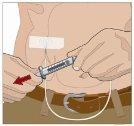
SI se utiliza el método de empuje para administrar (HY):
SI se utiliza el método de bombeo para administrar (HY):
|
|
|
|
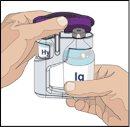
Siga las instrucciones del fabricante a la hora de preparar la bomba. | |
|
|
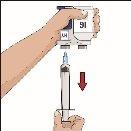
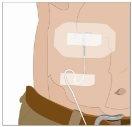
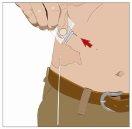
| Ángulo de 90º en relación con la piel
|
|
|
|
|
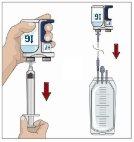
Divida el contenido a partes iguales entre todos los puntos, si se utiliza más de un punto. Si se utiliza el método de empuje para administrar HY:
Si se utiliza el método de bombeo para administrar HY:
|
|
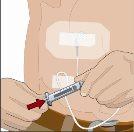
Tras perfundir todo el contenido de la jeringa más pequeña (hialuronidasa humana recombinante), retire la jeringa del conector de la aguja o de los tubos de la bomba. Acople los tubos de la bomba al recipiente o al vial de Ig o la jeringa más grande que contiene la inmunoglobulina humana normal 10% a la aguja. Administre la inmunoglobulina humana normal 10% con una bomba a las velocidades indicadas por su profesional sanitario e inicie la perfusión. | |
| |
|
|
|
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Algunos efectos adversos, como cefalea, escalofríos o dolores corporales, se pueden reducir disminuyendo la velocidad de perfusión
Efectos adversos graves
La perfusión de medicamentos como HyQvia, en ocasiones, puede provocar reacciones alérgicas graves aunque raras. Puede experimentar una bajada repentina de la presión arterial y, en casos aislados, shock anafiláctico. Los médicos están al corriente de estos posibles efectos adversos y le controlarán durante y tras las perfusiones iniciales.
Los signos o síntomas típicos incluyen:
sensación de aturdimiento, mareo, o pérdida de conocimiento, erupción cutánea y picor, hinchazón de la boca o garganta, dificultad para respirar, sibilancia (sonido silbante que se produce al respirar), frecuencia cardiaca anormal, dolor en el pecho, tono azulado de labios o dedos de manos y pies, visión borrosa.
- Si nota cualquiera de los siguientes signos durante la perfusión, informe inmediatamente a su médico o al enfermero.
- Cuando utilice HyQvia en casa, deberá llevar a cabo la perfusión en presencia de un cuidador asignado que le ayudará a vigilar las reacciones alérgicas, a detener la perfusión y a solicitar ayuda si fuera necesario.
- Consulte también la sección 2 de este prospecto sobre el riesgo de reacciones alérgicas y el uso de HyQvia en casa.
Efectos adversos muy frecuentes (pueden ocurrir en más de 1 de cada 10 perfusiones):
Reacciones locales en el punto de perfusión (se incluyen todos los puntos de perfusión enumerados a continuación). Estas reacciones suelen desaparecer en unos pocos días.
Efectos adversos frecuentes (pueden ocurrir en hasta 1 de cada 10 perfusiones):
- dolor de cabeza
- malestar (náuseas)
- dolor abdominal/dolor a la palpación del abdomen
- enrojecimiento de la piel (eritema)
- reacciones en el punto de perfusión, incluidas dolor, molestias, dolor a la palpación, enrojecimiento, hinchazón y picor
- sensación de calor, fiebre
- debilidad (astenia), cansancio (fatiga), falta de energía (letargo) y sensación de malestar general
Efectos adversos poco frecuentes (pueden ocurrir en hasta 1 de cada 100 perfusiones):
- mareo
- migraña
- sensaciones de entumecimieno, hormigueo, agujetas (parestesia)
- temblor
- latido cardiaco rápido (taquicardia)
- presión arterial alta (hipertensión)
- hinchazón del estómago (distensión abdominal)
- diarrea
- vómitos
- erupción
- picor (prurito)
- erupción con picor (urticaria)
- dolor muscular (mialgia)
- dolor articular (artralgia)
- dolor de espalda
- dolor en las extremidades (incluidas molestias en las extremidades)
- dolor torácico musculoesquelético
- rigidez articular
- reacciones en el punto de la perfusión (como cambio de color, contusión, enrojecimiento [hematoma], sangrado [hemorragia], punción del vaso sanguíneo, masa [nódulo], induración, hinchazón [edema], escalofríos, sensación de ardor, erupción)
- hinchazón genital
- Efectos adversos raros (pueden ocurrir hasta en 1 de cada 1 000 perfusiones):
- ictus
- presión arterial baja (hipotensión)
- dificultad para respirar (disnea)
- dolor inguinal
- orina marrón (hemosiderinuria)
- sudoración excesiva (hiperhidrosis)
- inflamación del punto de perfusión
- calor en el punto de perfusión
- sensaciones de entumecimieno, hormigueo y agujetas en el punto de perfusión (parestesia en el punto de perfusión)
- resultado positivo en la prueba de Coombs
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- inflamación de las membranas que rodean el cerebro y la médula espinal (meningitis aséptica)
- reacciones alérgicas (hipersensibilidad)
- pérdida en el punto de perfusión
- síndrome pseudogripal (enfermedad de tipo gripal)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de HyQvia
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC - 8 ºC). No congelar.
No agitar.
Conservar los viales en el embalaje exterior para protegerlos de la luz.
No utilice este medicamento si observa que las soluciones tienen aspecto turbio o si tienen partículas o sedimentos.
Tras su apertura, deseche cualquier resto de solución no utilizada de los viales.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de HyQvia
HyQvia es una unidad de vial doble que contiene:
- una solución de hialuronidasa humana recombinante (Paso 1 de HyQvia/Perfundir primero) y
- una solución de inmunoglobulina humana normal 10 % (Paso 2 de HyQvia/Perfundir segundo).
El contenido de cada vial se describe a continuación:
- Hialuronidasa humana recombinante
Este vial contiene hialuronidasa humana recombinante.
Los demás componentes son cloruro de sodio, fosfato de sodio, albúmina humana, ácido etilendiaminotetraacético (EDTA) disódico, cloruro de calcio y agua para preparaciones inyectables (ver también sección 2, “HyQvia contiene sodio”).
- Inmunoglobulina humana normal 10%
Un ml de la solución de este vial contiene 100 mg de inmunoglobulina humana normal de la que, al menos, el 98% es inmunoglobulina G (IgG).
El principio activo de HyQvia es inmunoglobulina humana normal. Este medicamento contiene trazas de inmunoglobulina A (IgA) (no más de 140 microgramos/ml, 37 microgramos de media).
Los demás componentes de este vial son glicina y agua para preparaciones inyectables.
Aspectodel productoy contenido del envase
HyQvia 100 mg/ml solución para perfusión por vía subcutánea (perfusión bajo la piel).
HyQvia se suministra en un envase que contiene:
- un vial de vidrio de hialuronidasa humana recombinante y
- un vial de vidrio de inmunoglobulina humana normal 10%.
La hialuronidasa humana recombinante es una solución transparente e incolora.
La inmunoglobulina humana normal 10% es una solución transparente e incolora o ligeramente amarillenta.
Están disponibles los siguientes tamaños de envase:
Hialuronidasa humana recombinante | Inmunoglobulina humana normal10% | |
Volumen (ml) | Proteína (g) | Volumen (ml) |
1,25 | 2,5 | 25 |
2,5 | 5 | 50 |
5 | 10 | 100 |
10 | 20 | 200 |
15 | 30 | 300 |
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización yresponsablede la fabricación
Titular de la autorización de comercialización:
Baxalta Innovations GmbH
Industriestrasse 67
A‑1221 Viena
Austria
Responsable de la fabricación:
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B‑7860 Lessines
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Takeda Farmacéutica España, S.A
Tel: +34 917 90 42 22
Fecha de la última revisión de este prospecto: 05/2024.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HYQVIA 100 MG/ML SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE, 165 mg/mlPrincipio activo: immunoglobulins, normal human, for extravascular adm.Fabricante: Octapharma S.A.Requiere recetaForma farmacéutica: INYECTABLE, 200 mg/mlPrincipio activo: immunoglobulins, normal human, for extravascular adm.Fabricante: Baxalta Innovations GmbhRequiere recetaForma farmacéutica: INYECTABLE, 200 mg/mlPrincipio activo: immunoglobulins, normal human, for extravascular adm.Fabricante: Csl Behring GmbhRequiere receta
Médicos online para HYQVIA 100 MG/ML SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HYQVIA 100 MG/ML SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes