HIZENTRA 200 mg/mL Subcutaneous Injectable Solution

How to use HIZENTRA 200 mg/mL Subcutaneous Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Hizentra 200 mg/ml Solution for Subcutaneous Injection
human normal immunoglobulin (IgSC = Immunoglobulin Subcutaneous)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Hizentra is and what it is used for
- What you need to know before you use Hizentra
- How to use Hizentra
- Possible side effects
- Storage of Hizentra
- Contents of the pack and other information
1. What Hizentra is and what it is used for
What Hizentra is
Hizentra belongs to a class of medicines called human normal immunoglobulins. Immunoglobulins are also known as antibodies and are proteins in the blood that help the body fight infections.
How Hizentra works
Hizentra contains immunoglobulins that have been obtained from the blood of healthy people. Immunoglobulins are produced by the body's immune system. They help the body fight infections caused by bacteria and viruses or maintain balance in the immune system (known as immunomodulation). The medicine works exactly like the immunoglobulins that are naturally present in the blood.
What Hizentra is used for
Replacement therapy
Hizentra is used to raise abnormally low levels of immunoglobulin in the blood to normal levels (replacement therapy). The medicine is used in adults and children (0-18 years) in the following situations:
- Treatment of patients born with a reduced ability or inability to produce immunoglobulins (primary immunodeficiencies). This includes conditions such as:
- Low levels of immunoglobulin (hypogammaglobulinemia) or absence of immunoglobulins in the blood (agammaglobulinemia)
- Combination of low levels of immunoglobulins, frequent infections, and inability to produce adequate levels of antibodies after vaccination (common variable immunodeficiency)
- Combination of low or absent levels of immunoglobulins and absence or non-functionality of immune cells (severe combined immunodeficiency)
- Lack of certain subclasses of immunoglobulin G that cause recurrent infections.
- Treatment of patients with low or dysfunctional levels of immunoglobulin in acquired conditions (secondary immunodeficiency) who experience severe or recurrent infections due to a weakened immune system as a result of other conditions or treatments.
Immunomodulatory therapy in patients with CIDP
Hizentra is also used in patients with chronic inflammatory demyelinating polyneuropathy (CIDP), a type of autoimmune disease. CIDP is characterized by chronic inflammation of the peripheral nerves that cause muscle weakness and/or numbness mainly in the legs and arms. It is believed that the body's attack on itself exacerbates this inflammation, and the immunoglobulins in Hizentra help protect the nerves from this attack (immunomodulatory therapy).
2. What you need to know before you use Hizentra
DO NOTinfuse Hizentra if:
- You are allergic to human immunoglobulins, polysorbate 80, or L-proline.
- Tell your doctor or healthcare professional before treatment if you have had any intolerance to any of these components in the past.
- You have hyperprolinemia (a genetic disorder caused by high levels of the amino acid proline in the blood).
In blood vessels.
Warnings and precautions
- Consult your doctor, pharmacist, or nurse before using Hizentra.
You may be allergic (hypersensitive) to immunoglobulins without knowing it. However, true allergic reactions are rare. They can occur even if you have previously received human immunoglobulins and tolerated them well. This can happen especially if you do not have enough immunoglobulin A (IgA) in your blood (IgA deficiency).
- Tell your doctor or healthcare professional if you have an IgA deficiency before starting treatment. Hizentra contains residual amounts of IgA that can cause allergic reactions.
In these rare cases, allergic reactions can occur, such as a sudden drop in blood pressure or shock(see also section 4 "Possible side effects").
- If you notice symptoms of this type during the infusion of Hizentra, stop the infusion and contact your doctor or go to the nearest hospital immediately.
- Tell your doctor if you have a history of heart or vascular disease, or if you have had blood clots, have thick blood, or have been immobilized for some time. These factors can increase your risk of having a blood clot after taking Hizentra. Also, tell your doctor what medications you are taking, as some contain estrogens (a hormone found, for example, in birth control pills) that can increase your risk of developing a blood clot. Contact your doctor immediately if you experience signs and symptoms such as difficulty breathing, chest pain, pain and swelling of a limb, weakness, or numbness of one side of the body after taking Hizentra.
- Contact your doctor if you experience the following signs and symptoms: severe headache, stiff neck, drowsiness, fever, photophobia, nausea, and vomiting after taking Hizentra. Your doctor will decide if further tests are needed and if you should continue treatment with Hizentra.
Your healthcare professional will avoid possible complications by checking that:
- You are not sensitive to human normal immunoglobulin.
The product must be injected at a slow initial rate. The recommended injection rate in section 3 "How to use Hizentra" must be strictly respected.
- Any symptoms are closely monitored during the entire infusion period, especially if:
you are receiving human normal immunoglobulin for the first time
- you have changed from another treatment
- a prolonged period (more than 8 weeks) has passed since your last infusion.
In these cases, you should be observed during the first infusion and for one hour after. If the above points do not apply to you, it is recommended that you be observed for at least 20 minutes after administration.
Interaction of Hizentra with other medicines
- Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
- Do not mix Hizentra with other medicines.
- Before vaccination, tell your doctor about your treatment with Hizentra.
Hizentra may alter the effect of some live virus vaccines such as measles, mumps, rubella, and varicella vaccine. Therefore, after administration of these medicines, you should wait at least 3 months before receiving a live attenuated vaccine. In the case of the measles vaccine, this alteration may persist for up to 1 year.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist. Your doctor will decide if you can receive Hizentra during pregnancy or while breastfeeding.
No studies have been conducted with Hizentra in pregnant women. However, medicines containing immunoglobulin have been used in pregnant or breastfeeding women for many years, and no adverse effects have been observed in the course of pregnancy or in the baby.
If you are breastfeeding and receive Hizentra, the immunoglobulins in the medicine may also be found in breast milk. Therefore, your baby may be protected against some infections.
Driving and using machines
During treatment with Hizentra, patients may experience effects such as dizziness or nausea that could affect the ability to drive and use machines. If this happens, do not drive or use machines until these effects have disappeared.
Hizentra contains proline
Do not take this medicine if you have hyperprolinemia (see also section 2 "What you need to know before you use Hizentra"). Tell your doctor before treatment.
Other important information about Hizentra
Blood tests
After receiving Hizentra, the results of some blood tests (serological tests) may be altered for some time.
- Before having any blood tests, tell your doctor about your treatment with Hizentra.
Information about the components of Hizentra
Hizentra is made from human blood plasma (the liquid part of the blood). When medicines are made from blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These measures include:
- a careful selection of blood or plasma donors to ensure the exclusion of potential carriers of infections, and
- examination of each donation and plasma pools for the absence of signs of viruses or infections.
Manufacturers of these medicines also include steps in the processing of blood or plasma that can inactivate or remove viruses. Despite these measures, when administering medicines prepared from human blood or plasma, the possibility of transmitting an infection cannot be completely excluded. This is also true for any unknown or emerging virus or other type of infection.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV, the AIDS virus), hepatitis B virus, and hepatitis C virus (inflammation of the liver), and for non-enveloped viruses such as hepatitis A virus and parvovirus B19.
- It is strongly recommended that, each time you receive a dose of Hizentra, you note the name and batch number of the product, in order to maintain a record of the batches used (see section 3, "How to use Hizentra").
Hizentra contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per vial/syringe; i.e., it is essentially "sodium-free".
3. How to use Hizentra
Follow your doctor's administration instructions for this medication exactly. Consult your doctor if you have any doubts.
Dose
Your doctor will decide how much Hizentra you will receive, based on your weight and response to treatment.
The dose or administration interval should not be changed without consulting your doctor.
If you think you should receive Hizentra more or less frequently, please consult your doctor. If you think you have missed a dose, talk to your doctor as soon as possible.
Replacement therapy
Your doctor will determine if you need a loading dose (for adults and children) of at least 1 to 2.5 ml/kg of body weight divided over several days. After that, maintenance doses will be administered at repeated intervals, from once a day to once every two weeks, to achieve a monthly accumulated dose of between 2 and 4 ml/kg of body weight. Your healthcare professional may adjust the dose based on your response to treatment.
Immunomodulatory therapy
Your doctor will start therapy with Hizentra one week after your last intravenous infusion of immunoglobulin, administering it subcutaneously with a weekly dose of 1.0 to 2.0 ml/kg of body weight. Your doctor will determine your weekly dose of Hizentra. The weekly maintenance doses can be divided into smaller doses and administered as required during the week. For bi-weekly schedules, your doctor will double the weekly dose of Hizentra. Your healthcare professional may adjust the dose based on your response to treatment.
Form and route of administration
In the case of home treatment, this must be initiated by a healthcare professional with experience in the treatment of immunodeficiency/PID with SCIG and in instructing patients for home treatment.
You will be instructed and trained in:
- aseptic infusion techniques,
- maintaining a treatment diary, and
- measures to take in case of serious adverse effects.
Infusion point(s)
- Administer Hizentra only by subcutaneous route.
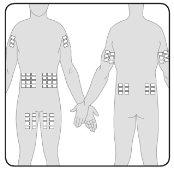
- You can infuse Hizentra at points such as the abdomen, thigh, arm, and lateral hip. In case of high doses (> 50 ml), administer at multiple points.
- You can use an unlimited number of infusion points simultaneously. The points where injections are applied must be at least 5 cm apart.
- If you use the assisted infusion device technique (e.g., pump-assisted infusion), you can use more than one infusion device simultaneously.
- If you use the manual push technique with a syringe, you can only use one infusion site per syringe. If you need to administer an additional syringe of Hizentra, you must use a new sterile needle and change the infusion site.
- The volume of product infused at a given site may vary.
Infusion rate(s)
Your doctor will determine the appropriate infusion technique and infusion rate for you, taking into account your individual dose, dosing frequency, and product tolerability.
Device-assisted infusion:
The recommended initial infusion rate is up to 20 ml/hour per point. If it is well tolerated, the infusion rate can be gradually increased to 35 ml/hour per point for the next two infusions. From then on, the infusion rate can be increased according to your tolerability.
Manual push infusion:
The recommended initial infusion rate is up to 0.5 ml/min per point (30 ml/hour per point). If it is well tolerated, the infusion rate can be increased up to 2.0 ml/min per point (120 ml/hour per point) for subsequent infusions. From then on, the infusion rate can be increased further according to your tolerability.
Instructions for use
Follow these steps and use an aseptic technique to administer Hizentra. | ||
1 | Clean the surface Clean the table or other flat surface thoroughly using an antiseptic wipe. | |
2 | Assemble the accessories Place Hizentra and the rest of the necessary accessories and equipment for infusion on a clean and flat surface. | |
3 | Wash and dry your hands thoroughly | |
4 | Inspect the vials Visually inspect Hizentra for the presence of particles in the solution or discoloration of the solution, as well as the expiration date before administering Hizentra. Do not use cloudy or particle-containing solutions. Do not use solutions that have been frozen. Administer the solution when it is at room temperature or body temperature. Once the vial is opened, use the solution immediately. | |
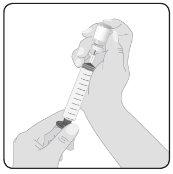
5 | Prepare Hizentra for infusion Clean the vial stopper– Remove the protective cap from the vial and expose the central portion of the rubber stopper. Clean the stopper with an alcohol wipe or antiseptic preparation and let it dry. Transfer Hizentra to the infusion syringe– Attach the transfer device or needle to a sterile syringe, using an aseptic technique. If using a transfer device (vented spike), follow the manufacturer's instructions. If using a needle, pull the plunger back to draw air into the syringe in a quantity similar to the amount of Hizentra to be withdrawn. Then, insert the needle into the center of the vial stopper and, avoiding foam formation, inject the air into the air chamber of the vial (not into the liquid). Finally, withdraw the desired volume of Hizentra. If using multiple vials to achieve the desired dose, repeat this step. |
|
6 | Prepare the connector Attach the administration connector or needle equipment to the syringe. Prime the connector to eliminate any remaining air. | |
7 | Prepare the infusion point(s) Select the infusion point(s) –The number and location of the infusion points depend on the total dose volume. Each infusion point must be at least 5 cm apart. You can use an unlimited number of infusion points simultaneously. |
|
Clean the infusion point(s)using an antiseptic skin preparation. Let each point dry before proceeding. | ||
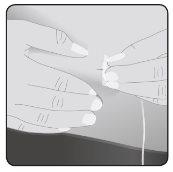
8 | Insert the needle Take the skin between 2 fingers and insert the needle into the subcutaneous tissue. |
|
Secure the needle to the skin– If necessary, use gauze and tape or a transparent dressing to secure the needle in place. | ||
9 | Infuse Hizentra Start the infusion. If you are using an infusion pump, follow the manufacturer's instructions. | |
10 | Record the infusion Record the following data in your treatment diary:
| |
11 | Cleaning Discard unused product and all used administration accessories after administration, in accordance with local requirements. |
If you have any doubts about the use of this product, consult your doctor, pharmacist, or nurse.
If you use more Hizentra than you should
Inform your doctor as soon as possible if you think you have received too much Hizentra.
If you miss a dose of Hizentra
Inform your doctor as soon as possible if you think you have missed a dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
- In isolated cases, you may be allergic (hypersensitive) to immunoglobulins and may experience allergic reactions such as a sudden drop in blood pressure or shock (e.g., you may feel dizzy or lightheaded, feel dizzy when standing up, have cold hands and feet, an abnormal heart rhythm or chest pain, or have blurred vision).
- In isolated cases, you may experience pain and/or swelling of an arm or leg with heat in the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens with deep breathing, rapid heart rate, numbness or weakness on one side of the body, sudden confusion or difficulty speaking or understanding, which could be signs of a blood clot.
- In isolated cases, you may have a severe headache with nausea, vomiting, stiff neck, fever, and sensitivity to light, which could be signs of aseptic meningitis syndrome (AMS), a temporary, reversible, non-infectious inflammation of the membranes surrounding the brain and spinal cord.
- If you notice such signs during the infusion of Hizentra, interrupt the infusion and go to the nearest hospital immediately.
See also section 2 of this leaflet on the risk of allergic reactions, blood clots, and AMS.
The side effects observed in controlled clinical trials are presented in decreasing order of frequency. The side effects observed post-marketing are of unknown frequency:
The following side effects are very common(affect more than 1 in 10 patients):
- Headache
- Rash
- Infusion site reactions
The following side effects are common(affect between 1 and 10 patients in 100):
- Dizziness
- Migraine
- Increased blood pressure (hypertension)
- Diarrhea
- Abdominal pain
- Malaise (nausea)
- Vomiting
- Itching (pruritus)
- Hives (urticaria)
- Musculoskeletal pain (musculoskeletal pain)
- Fever
- Joint pain (arthralgia)
- Fatigue (fatigue), including malaise (general discomfort)
- Chest pain
- Flu-like symptoms
- Pain
The following side effects are uncommon(affect between 1 and 10 patients in 1,000):
- Hypersensitivity
- Involuntary movements in one or more parts of the body (tremors, including psychomotor hyperactivity)
- Rapid heart rate (tachycardia)
- Flushing
- Muscle spasms
- Muscle weakness
- Chills, including low body temperature
- Abnormal blood test results that may indicate liver and kidney function disorders
In isolated cases, an ulcer may occur at the infusion site or a burning sensation.
- You can reduce possible side effects if you infuse Hizentra slowly.
These side effects may occur even if you have previously received human immunoglobulins and tolerated them well.
Also, consult section 2 "What you need to know before you start using Hizentra", where you will find additional information on factors that may increase the risk of side effects.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hizentra
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiration date stated on the packaging and on the vial label after EXP.
- You must use/infuse this medicine as soon as possible after opening the vial. Do not use Hizentra if the vial is open or defective.
- Do not store above 25 °C.
- Do not freeze.
- Keep the vial in the outer packaging to protect it from light.
- Do not throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. This will help protect the environment.
6. Package contents and additional information
Composition of Hizentra
- The active substanceis normal human immunoglobulin. One ml contains 200 mg of normal human immunoglobulin, of which at least 98% is immunoglobulin G (IgG).
The approximate percentage of IgG subclasses is as follows:
IgG1 .................................. 69%
IgG2 .................................. 26%
IgG3 .................................. 3%
IgG4 .................................. 2%
This medicine contains traces of IgA (no more than 50 micrograms/ml).
- The other ingredients(excipients) are L-proline, polysorbate 80, and water for injectable preparations.
Appearance of Hizentra and package contents
Hizentra is a subcutaneous injectable solution (200 mg/ml). The color may vary from pale yellow to light brown.
Hizentra is available in vials of 5, 10, 20, or 50 ml.
Hizentra is also available in pre-filled syringes of 5, 10, 20, and 50 ml.
Package sizes
Packs of 1, 10, or 20 vials.
Hizentra is also available in packs of 1 (for 5, 10, 20, 50 ml) or 10 (for 5, 10, 20 ml) or 20 (for 10, 20 ml) pre-filled syringes.
Please note that this packaging does not contain alcohol wipes, needles, or other accessories or equipment.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
D-35041 Marburg
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien CSL Behring NV Tél/Tel: +32 15 28 89 20 | Luxembourg/Luxemburg CSL Behring NV Tél/Tel: +32 15 28 89 20 |
| Magyarország CSL Behring Kft. Tel: +36 1 213 4290 |
Ceská republika CSL Behring s.r.o. Tel: + 420 702 137 233 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Danmark CSL Behring AB Tel: +46 8 544 966 70 | Nederland CSL Behring BV Tel: + 31 85 111 96 00 |
Deutschland CSL Behring GmbH Tel: +49 69 30584437 | Norge CSL Behring AB Tlf: +46 8 544 966 70 |
Eesti CentralPharma Communications OÜ Tel: +3726015540 | Österreich CSL Behring GmbH Tel: +43 1 80101 2463 |
Ελλάδα CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Polska CSL Behring Sp. z o.o. Tel: +48 22 213 22 65 |
España CSL Behring S.A. Tel: +34 933 67 1870 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
France CSL Behring SA Tél: + 33 1 53 58 54 00 | România Prisum Healthcare S.R.L. Tel: +40 21 322 01 71 |
Hrvatska Marti Farm d.o.o. Tel: +385 1 5588297 | Slovenija EMMES BIOPHARMA GLOBAL s.r.o. - podružnica v Sloveniji Tel: +386 41 42 0002 |
Ireland CSL Behring GmbH Tel: +49 69 305 17254 | Slovenská republika CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Ísland CSL Behring AB Sími: +46 8 544 966 70 | Suomi/Finland CSL Behring AB Puh/Tel: +46 8 544 966 70 |
Italia CSL Behring S.p.A. Tel: +39 02 34964 200 | Sverige CSL Behring AB Tel: +46 8 544 966 70 |
Κύπρος CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | United Kingdom (Northern Ireland) CSL Behring GmbH Tel: +49 69 30517254 |
Latvija CentralPharma Communications SIA Tel: +371 6 7450497 | |
Lietuva CentralPharma Communications UAB Tel: +370 5 243 0444 |
Date of the last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/.
----------------------------------------------------------------------------------------------------------------
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HIZENTRA 200 mg/mL Subcutaneous Injectable SolutionDosage form: INJECTABLE, 165 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Baxalta Innovations GmbhPrescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Csl Behring GmbhPrescription required
Online doctors for HIZENTRA 200 mg/mL Subcutaneous Injectable Solution
Discuss questions about HIZENTRA 200 mg/mL Subcutaneous Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions