
HEMANGIOL 3,75 MG/ML SOLUCION ORAL

Cómo usar HEMANGIOL 3,75 MG/ML SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
HEMANGIOL 3,75 mg/ml, solución oral
propranolol
Lea todo el prospecto detenidamente antes de que su hijo empiece a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a su hijo, y no debe dárselo a otras personas aunque tengan los mismos síntomas que su hijo, ya que puede perjudicarles.
- Si su hijo experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es HEMANGIOL y para qué se utiliza
- Qué necesita saber antes de que su hijo empiece a tomar HEMANGIOL
- Cómo tomar HEMANGIOL a su hijo
- Posibles efectos adversos
- Conservación de HEMANGIOL
- Contenido del envase e información adicional
1. Qué es HEMANGIOL y para qué se utiliza
Qué es HEMANGIOL
El nombre de su medicamento es HEMANGIOL. El principio activo es propranolol.
El propranolol pertenece a un grupo de medicamentos conocidos como betabloqueantes.
Para qué se utiliza
Este medicamento se utiliza para tratar una enfermedad llamada hemangioma. Un hemangioma es un acúmulo extra de vasos sanguíneos que han formado un bulto en la piel o debajo de la misma. El hemangioma puede ser superficial o profundo. A veces se llama “marca de fresa” porque la superficie de un hemangioma se parece un poco a una fresa.
Hemangiol se inicia en niños que tienen desde 5 semanas hasta 5 meses de edad, cuando:
- la localización y/o la extensión de las lesiones ponen en peligro la vida o la función de un órgano (podrían alterar órganos vitales o sentidos como la visión o la audición);
- el hemangioma está ulcerado (es decir, llaga en la piel que no se cura) y es doloroso, y/o no responde a medidas básicas de cuidado de heridas;
- hay riesgo de cicatrices permanentes o desfiguración.
2. Qué necesita saber antes de que su hijo empiece a tomar HEMANGIOL
No use HEMANGIOL
Si su hijo:
- ha nacido prematuramente y no ha alcanzado la edad corregida de 5 semanas (la edad corregida es la edad que tendría un lactante prematuro si hubiera nacido en la fecha prevista);
- es alérgico al propranolol o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6). Una reacción alérgica puede incluir una erupción cutánea, picor o dificultad respiratoria;
- tiene asma o antecedentes de dificultades respiratorias;
- tiene una frecuencia cardíaca lenta para su edad. Debe consultar con su médico si no está seguro/a;
- tiene un problema cardíaco (como trastornos del ritmo cardíaco e insuficiencia cardíaca);
- tiene una presión arterial muy baja;
- tiene problemas circulatorios que hacen que los dedos de los pies y de las manos estén entumecidos y pálidos;
- tiene propensión a una concentración baja de azúcar en sangre;
- tiene la presión arterial elevada por un tumor de la glándula suprarrenal. Esto se llama “feocromocitoma”.
Si usted está dando de mamar a su hijo y si está tomando medicamentos que no se deben utilizar con HEMANGIOL (ver “Si está dando de mamar a su hijo” y “Uso de HEMANGIOL con otros medicamentos”), no administreeste medicamento a su hijo.
Advertencias y precauciones
Antes de que su hijo empiece a tomar HEMANGIOL, informe a su médico:
- si su hijo tiene problemas de hígado o riñones. Este medicamento no se recomienda en caso de insuficiencia hepática o renal;
- si su hijo ha tenido alguna vez una reacción alérgica, cualquiera que sea su origen (por ejemplo, medicamentos o sustancias alimenticias, etc.). Una reacción alérgica puede incluir una erupción cutánea, picor o dificultad respiratoria;
- si su hijo tiene psoriasis (una enfermedad de la piel que produce placas rojas y secas de piel endurecida), porque este medicamento puede empeorar los síntomas de esta enfermedad;
- si su hijo tiene diabetes: en este caso se debe aumentar la frecuencia de monitorización de la glucosa sanguínea de su hijo.
- si su hijo tiene síndrome PHACE (un trastorno que combina hemangioma y malformaciones vasculares que pueden afectar a los vasos sanguíneos cerebrales), porque este medicamento puede aumentar el riesgo de ictus cerebral.
Signos importantes que se deben buscar después de la administración de HEMANGIOL
Riesgos de hipoglucemia
Este medicamento puede enmascarar los signos de alarma de la hipoglucemia (también conocida como concentración baja de azúcar en sangre). También puede agravar la hipoglucemia en niños, especialmente durante el período de ayunas (p.ej., mala ingesta de alimentos por vía oral, infección, vómitos), cuando aumentan las demandas de glucosa (resfriado, estrés, infecciones), o en caso de sobredosis. Estos signos pueden ser:
- Leves: palidez, cansancio, sudoración, temblor, palpitaciones, ansiedad, hambre, dificultad para levantarse.
- Graves: somnolencia excesiva, dificultad para responder, problemas para alimentarle, disminución de la temperatura corporal, convulsiones (crisis), pausas breves en la respiración, pérdida de la conciencia.
El riesgo de desarrollar hipoglucemia se mantiene elevado durante todo el período de tratamiento.
Para evitar los riesgos de hipoglucemia,debe administrar HEMANGIOL durante o inmediatamente después de una comida y evitar administrar la última dosis cerca de la hora de acostarse por la noche (ver sección 3).Debe alimentarsuficientemente ycon frecuencia a su hijo durante el tratamiento. Si su hijo no come suficiente, si presenta otra enfermedad o si vomita, se recomienda omitir la dosis. NO ADMINISTRE HEMANGIOL A SU HIJO HASTA QUE SE HAYA ALIMENTADO CORRECTAMENTE. Si su hijo tiene algún signo de hipoglucemia mientras toma HEMANGIOL,interrumpa el tratamiento y llame rápidamente a su médico o vaya directamente al hospital. Si el niño está consciente,adminístrele un líquido oral que contenga azúcar. |
Riesgos de broncoespasmo
Interrumpa el tratamiento y contacte inmediatamente con un médico si después de administrar HEMANGIOL a su hijo observa los siguientes síntomas indicativos de broncoespasmo (restricción transitoria de los conductos bronquiales que produce dificultad respiratoria): tos, respiración rápida o difícil o sibilancias, asociados o no a piel de color azulado.
Interrumpa el tratamiento y contacte inmediatamente a un médico si su hijo tiene síntomas similares al resfriado asociados con dificultad para respirar y/o sibilancias mientras tomaHEMANGIOL. |
Riesgo de hipotensión y bradicardia (frecuencia cardíaca lenta)
HEMANGIOL puede reducir la presión arterial (hipotensión) y la frecuencia cardíaca (bradicardia). Por este motivo, su hijo debe estar bajo una estrecha monitorización clínica y de la frecuencia cardíaca durante 2 horas después de la primera toma y después de un aumento de la dosis. Posteriormente, durante el tratamiento su médico le realizará exploraciones clínicas periódicas a su hijo.
Interrumpa el tratamiento y contacte inmediatamente a un médico si su hijo tiene cualquier signo como cansancio, sensación de frío, palidez, coloración azulada de la piel o desvanecimiento mientras tomaHEMANGIOL. |
Riesgo de hiperpotasemia
HEMANGIOL puede aumentar la concentración sanguínea de potasio (hiperpotasemia). En caso de hemangioma ulcerado grande, se debe medir la concentración sanguínea de potasio de su hijo.
Si a su hijo se le va a administrar anestesia general
Informe a su médico de que su hijo está tomando HEMANGIOL. Esto se debe a que su hijo puede tener la presión arterial baja si se le administran determinados anestésicos mientras toma este medicamento (ver “Uso de HEMANGIOL con otros medicamentos”). Podría ser necesario interrumpir HEMANGIOL al menos 48 horas antes de la anestesia.
Si está dando de mamar a su hijo
- Informe a su médico antes de administrar este medicamento.
- No administre este medicamento a su hijo si usted está tomando medicamentos que no se deben utilizar con HEMANGIOL (ver “Uso de HEMANGIOL con otros medicamentos”).
Uso de HEMANGIOL con otros medicamentos
- Informe a su médico, farmacéutico o enfermero si está administrando, ha administrado recientemente o podría tener que administrar cualquier otro medicamento a su hijo. Esto se debe a que HEMANGIOL puede modificar la forma en la que actúan otros medicamentos, y algunos medicamentos pueden influir en la forma en la que actúa HEMANGIOL.
- Además, si está dando de mamar a su hijo, es importante que informe a su médico, farmacéutico o enfermero de los medicamentos que está tomando usted, porque pueden pasar a la leche materna e interferir con el tratamiento de su hijo. Su médico le aconsejará si es necesario interrumpir la lactancia materna o no.
En particular, en caso de que esté dando de mamar a su hijo, informe a su médico o farmacéutico si usted o su hijo está tomando:
- medicamentos para la diabetes;
- medicamentos para problemas del corazón y de los vasos sanguíneos, como latidos cardíacos irregulares, dolor en el pecho o angina, presión arterial elevada o insuficiencia cardíaca;
- medicamentos para tratar la ansiedad y la depresión, además de problemas más graves de salud mental, y la epilepsia;
- medicamentos para tratar la tuberculosis;
- medicamentos para tratar el dolor y la inflamación;
- medicamentos utilizados para reducir las grasas en la sangre;
- medicamentos utilizados para la anestesia.
Si tiene cualquier otra pregunta, consulte con su médico o farmacéutico.
HEMANGIOL contiene sodio y propilenglicol
Este medicamento contiene menos de 23 mg de sodio (1mmol) por dosis; esto es, esencialmente “exento de sodio”.
Este medicamento contiene 2,08 mg de propilenglicol/kg/día. Si el bebé tiene menos de 4 semanas de edad, consulte a su médico o farmacéutico, en particular si al bebé se le han administrado otros medicamentos que contengan propilenglicol o alcohol.
3. Cómo administrar HEMANGIOL a su hijo
El tratamiento de su hijo ha sido iniciado por un médico que tiene experiencia en el diagnóstico, el tratamiento y el manejo del hemangioma infantil.
Siga exactamente las instrucciones de administración a su hijo de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Nunca modifique usted mismo la dosis que está administrando a su hijo. Todos los incrementos de dosis y todos los ajustes por el peso de su hijo los debe realizar su médico.
Dosis
- La dosis se basa en el peso de su niño siguiendo el siguiente esquema:
Semanas (dosis diaria) | Dosis por toma | Horas de las tomas |
Primera semana (1 mg/kg/día) | 0,5 mg/kg |
|
Segunda semana (2 mg/kg/día) | 1mg/kg | |
Tercera y semanas siguientes (3 mg/kg/día) | 1,5 mg/kg |
.
- Cuando sea necesario, puede diluir el medicamento en una pequeña cantidad de leche infantil o de zumo de manzana y/o de naranja adaptado para la edad del niño, y administrarlo a su hijo en un biberón. No mezcle el medicamento directamente con un biberón lleno de leche o zumo.
Para niños de hasta 5 kg de peso, puede mezclar la dosis con una cucharadita de leche infantil (aproximadamente 5 ml). Para niños de más de 5 kg de peso se puede mezclar la dosis con una cucharada de leche infantil o zumo de fruta (aproximadamente 15 ml).
Utilice la mezcla en las 2 horas siguientes a su preparación.
Cómo administrar HEMANGIOL a su hijo
- Hemangiol es para vía oral.
- El medicamento se debe administrar durante la alimentación del niño o inmediatamente después de la misma.
- La dosis siempre se debe medir con la jeringuilla oral que se suministra con el frasco.
- Administre HEMANGIOL directamente en la boca de su hijo utilizando la jeringa para uso oral que se incluye con el frasco.
- Alimente con frecuencia su hijo para evitar el ayuno prolongado.
- Si su hijo no come, o si vomita, se recomienda omitir la dosis.
- Si su hijo vomita una dosis, o si no está segura/o de que haya recibido todo el medicamento, no le administre otra dosis, simplemente espere hasta la siguiente dosis programada.
- Una misma persona debe administrar HEMANGIOL y la alimentación del niño a fin de evitar el riesgo de hipoglucemia. Si participan personas diferentes, es esencial una buena comunicación para garantizar la seguridad de su hijo.
Instrucciones de uso:
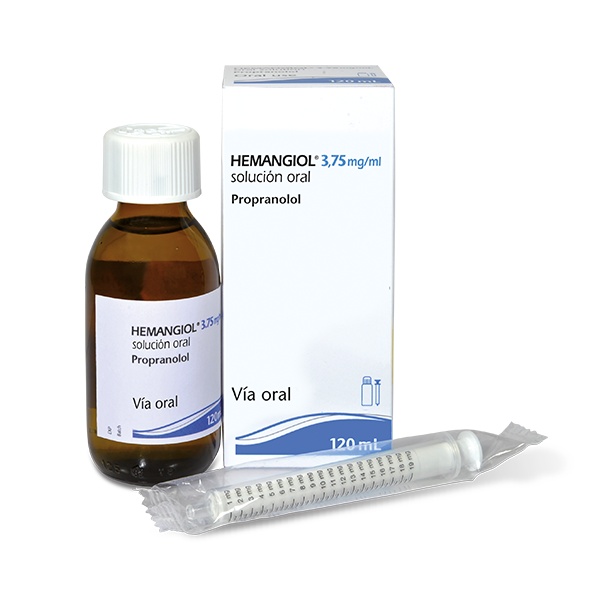
- Paso 1. Extraiga del estuche los diferentes elementos
El estuche contiene los siguientes elementosque necesitará para administrar el medicamento:
- El frasco de vidrio conteniendo 120 ml de solución oral de propranolol.
- La jeringa para uso oral dosificadora en mg que se incluye con este medicamento.
Extraer del estuche el frasco y la jeringa para uso oral, y saque la jeringa de la bolsa de plástico.
- Paso 2. Compruebe la dosis
Compruebe la dosis de HEMANGIOL en miligramos (mg) tal y como la ha recetado su médico.
Localice este número en la jeringa para uso oral.
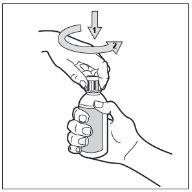
- Paso 3. Abra el frasco
El frasco tiene un tapón a prueba de niños. Esta es la manera de abrirlo: apriete hacia abajo el tapón de plástico a la vez que gira el tapón en sentido contrario a las agujas del reloj (hacia la izquierda).
No agite el frasco antes de su uso.
- Paso 4. Introduzca la jeringa
Introduzca la punta de la jeringa para uso oral en el frasco en posición vertical, y empuje el émbolo en todo su recorrido.
No extraiga el adaptador para la jeringa de la boca del frasco.
Utilice sólo la jeringa para uso oral que se suministra con el medicamento para medir y administrar la dosis. No utilice una cucharilla ni ningún otro dispositivo dispensador.
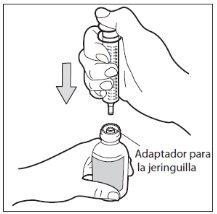
- Paso 5: Extraiga la dosis
Con la jeringa para uso oral colocada, gire el frasco para ponerlo boca abajo. Tire del émbolo de la jeringa hasta el número de mg que necesita.
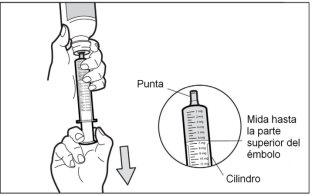
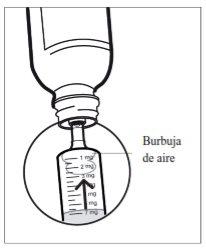
- Paso 6: Compruebe la ausencia de burbujas de aire
Si ve burbujas de aire en la jeringa, sujete la jeringa verticalmente, empuje el émbolo hacia arriba lo suficiente para expulsar por completo todas las burbujas de aire grandes, y reajústelo hasta la dosis prescrita por el médico.
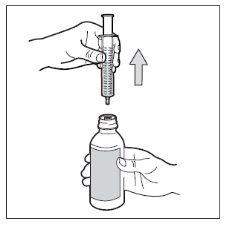
- Paso 7. Extraiga la jeringa
Gire el frasco para ponerlo en posición vertical, y separe del frasco toda la jeringa. Tenga cuidado de no empujar el émbolo durante este paso.
- Paso 8. Cierre el frasco
Vuelva a poner el tapón de plástico en el frasco girándolo en sentido de las agujas del reloj (hacia la derecha).
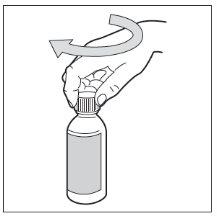
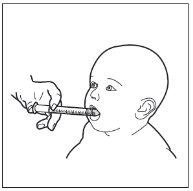
- Paso 9. Administre HEMANGIOL a su hijo
Introduzca la jeringa en la boca de su hijo y apóyela en la parte interna de la mejilla.
Ahora ya puede expulsar lentamente HEMANGIOL desde la jeringa directamente en la boca de su hijo.
No acueste al niño inmediatamente después de administrarle el medicamento.
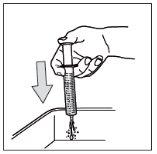
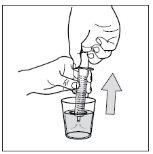
- Paso 10: Limpie la jeringa.
No desmonte la jeringa. Aclare la jeringa vacía en un vaso de agua limpia después de cada uso:
1- Tome un vaso de agua limpia
2- Tire del émbolo hasta el extremo
3- Tire el agua en el fregadero
4- Repita 3 veces este proceso de limpieza.
No utilice ningún producto con jabón o alcohol para limpiarla. Seque el exterior con un paño. No introduzca la jeringa en un esterilizador ni en un lavaplatos.
Guarde el frasco y la jeringa juntos en el estuche hasta el siguiente uso, en un lugar seguro en el que el niño no lo pueda ver o al que no pueda llegar. Tire la jeringa cuando se haya acabado el frasco.
Si administra a su hijo más HEMANGIOL del que debe
Si ha administrado a su hijo más HEMANGIOL del que debe, consulte inmediatamente con su médico.
Si olvidó administrar HEMANGIOL a su hijo
Omita la dosis olvidada, y no administre una dosis doble para compensar las dosis olvidadas. Continúe el tratamiento con la frecuencia habitual. Una dosis por la mañana y otra a última hora de la tarde.
Si interrumpe el tratamiento de su hijo con HEMANGIOL
HEMANGIOL puede dejar de administrarse de repente al final del tratamiento, según lo decida el médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Además, después de la administración de HEMANGIOL se deben buscar importantes signos de alarma de posibles efectos adversos como presión arterial baja, frecuencia cardíaca baja, concentración de azúcar en sangre baja o broncoespasmo (dificultades para respirar). Ver sección 2 de este prospecto.
Efectos adversosmuy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- bronquitis (inflamación de los bronquios),
- trastornos del sueño (insomnio, sueño de mala calidad y dificultad para despertarse),
- diarrea y vómitos.
Efectos adversosfrecuentes (pueden afectar hasta 1 de cada 10 personas):
- broncoespasmo (dificultad respiratoria),
- bronquiolitis (inflamación de bronquios pequeños con dificultad respiratoria y sibilancias en el pecho, asociada a tos y fiebre),
- disminución de la presión arterial,
- disminución del apetito,
- agitación, pesadillas, irritabilidad,
- somnolencia,
- extremidades frías,
- estreñimiento, dolor abdominal,
- eritema (enrojecimiento cutáneo).
- Dermatitis del pañal.
Efectos adversospoco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- trastornos de conducción o del ritmo cardíaco (latidos cardíacos lentos o irregulares),
- urticaria (reacción alérgica de la piel), alopecia (pérdida de cabello),
- disminución de la concentración de azúcar en sangre,
- reducción del número de leucocitos (células de la serie blanca en la sangre).
La frecuencia de los siguientes efectos adversos esno conocida (no se puede estimar a partir de los datos disponibles)
- convulsiones (crisis) relacionadas con la hipoglucemia (concentración anormalmente baja de glucosa en sangre),
- bradicardia (frecuencia cardíaca anormalmente baja),
- presión arterial baja,
- cifras muy bajas de leucocitos (células de la serie blanca en la sangre) que luchan contra las infecciones,
- problemas circulatorios que hacen que los dedos de los pies y de las manos estén entumecidos y pálidos,
- elevación de la concentración de potasio en sangre.
Comunicación de efectos adversos
Si su hijo experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante lacomunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de HEMANGIOL
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta del frasco. La fecha de caducidad es el último día del mes que se indica.
Conservar el frasco en el embalaje exterior para protegerlo de la luz. Guardar el frasco y la jeringa para uso oral en el estuche entre cada uso. No congelar.
Después de la primera apertura del frasco, el medicamento se debe usar en un plazo máximo de 2 meses.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y los medicamentos que ya no necesita. De esta manera, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de HEMANGIOL
- El principio activo es propranolol. Cada ml contiene 4,28 mg de hidrocloruro de propranolol, equivalente a 3,75 mg/ml de propranolol.
- Los demás ingredientes son hidroxietilcelulosa, sacarina sódica, aroma fresa (contiene propilenglicol), aroma vainilla (contiene propilenglicol), ácido cítrico monohidratado y agua purificada. Para más información, consulte la sección 2 en "HEMANGIOL contiene sodio y propilenglicol".
Aspecto de HEMANGIOL y contenido del envase
- HEMANGIOL es una solución oral transparente, incolora o de un color ligeramente amarillo, con olor afrutado.
- Se suministra en un frasco de vidrio de color ámbar de 120 ml, con un tapón de rosca a prueba de niños. Envase de 1 frasco.
- Con cada frasco se suministra una jeringa para uso oral de polipropileno dosificadora en mg de propranolol.
Titular de la autorización de comercialización
PIERRE FABRE MEDICAMENT
Les Cauquillous
81500 Lavaur
FRANCIA
Responsable de la fabricación
FARMEA
10 rue Bouché Thomas
ZAC Sud d’Orgemont
49000 ANGERS
FRANCIA
O
PIERRE FABRE MEDICAMENT PRODUCTION
Site PROGIPHARM, Rue du Lycée
45500 GIEN
FRANCIA
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Precio medio en farmacia225.59 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HEMANGIOL 3,75 MG/ML SOLUCION ORALForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: propranololFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 40 mgPrincipio activo: propranololFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: propranololFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para HEMANGIOL 3,75 MG/ML SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HEMANGIOL 3,75 MG/ML SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











