
GLIADEL 7,7 mg IMPLANTE

Cómo usar GLIADEL 7,7 mg IMPLANTE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
GLIADEL 7,7 mg implante
Carmustina
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es GLIADEL implante y para qué se utiliza
- Qué necesita saber antes de empezar a usar GLIADEL implante
- Cómo usar GLIADEL implante
- Posibles efectos adversos
- Conservación de GLIADEL implante
- Contenido del envase e información adicional
1. Qué es Gliadel implante y para qué se utiliza
GLIADEL implante es un sistema de liberación del principio activo anticancerígeno carmustina directamente en el lugar en el que se encontraba el tumor cerebral, tras su extirpación por cirugía. La carmustina pertenece a un grupo de sustancias anticancerígenas que permiten combatir el crecimiento de las células tumorales localizadas en el cerebro.
GLIADEL implante pueden usarse en combinación con la radioterapia para el tratamiento de tumores cerebrales.
Se ha demostrado que GLIADEL implante prolonga la supervivencia de los pacientes con tumor cerebral.
2. Qué necesita saber antes de empezar a usar Gliadel implante
No use GLIADEL implante
- si es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Tras la cirugía para extirpar el tumor cerebral e introducir GLIADEL implante, su médico o cirujano le vigilará de cerca por si hubiera complicaciones. En algunos casos, puede que el cirujano tenga que volver a operarle (debido a complicaciones o reaparición del tumor). Las complicaciones incluyen:
- Convulsiones (crisis)
- Infecciones en el cerebro (infecciones dentro del cráneo)
- Inflamación del cerebro debido a acumulación de líquidos
- Pérdida de fluido cerebral
- Problemas con la cicatrización de la herida
Su médico le controlará estrechamente en el caso de que esté tomando esteroides debido a una inflamación o presión elevada de fluidos en el cerebro.
Antes de introducir los implantes puede que el cirujano tenga que cerrar un canal en el cerebro para evitar que los implantes pasen a través de este, lo que podría provocar una acumulación de líquidos dentro del cráneo.
Tras la introducción de GLIADEL implante, mediante una técnica de obtención de imágenes, se puede detectar inflamación en el cerebro debido a la acumulación de líquidos e inflamación provocada por GLIADEL implante o progresión del tumor.
Otros medicamentos y GLIADEL implante
Informe a su médico si está utilizando o ha utilizando recientemente otros medicamentos, incluso los adquiridos sin receta.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. GLIADEL implante no se ha estudiado en mujeres embarazadas. Se ha demostrado que el principio activo, la carmustina, afecta negativamente al desarrollo del feto. GLIADEL implante no debe usarse en mujeres embarazadas o en periodo de lactancia. Se aconseja a las mujeres en edad fértil que utilicen métodos anticonceptivos efectivos durante 6 meses después de recibir GLIADEL implante. Los varones que tengan una pareja en edad fértil deben utilizar métodos anticonceptivos durante 90 días después de recibir GLIADEL implante.
Conducción y uso de máquinas
No se recomienda la conducción después del tratamiento. Consulte con su médico antes de conducir o usar herramientas o máquinas.
3. Cómo usar Gliadel implante
GLIADEL implante sólo debe usarse en adultos.
El cirujano o el farmacéutico se asegurarán de que el medicamento esté disponible para su operación. Tras la extirpación del tumor cerebral, el cirujano introducirá hasta ocho implantes en el espacio que ocupaba el tumor. Su cirujano decidirá el número de implantes que introducirá en la cavidad creada tras la extirpación del tumor cerebral. Los implantes se colocan de manera que cubran la mayor superficie posible de esta cavidad. Tras la operación, los implantes se disuelven lentamente a lo largo de dos a tres semanas, liberando así la carmustina directamente a las células circundantes.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su cirujano.
4. Posibles efectos adversos
Al igual que todos los medicamentos, GLIADEL puede producir efectos adversos, aunque no todas las personas los sufran.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico.
A continuación se presentan los efectos adversos más frecuentes observados durante los ensayos clínicos en pacientes con glioma maligno (tumor cerebral) de nuevo diagnóstico (120 pacientes) o glioma maligno recurrente diagnosticado (110 pacientes).
Las siguientes cuatro categorías de efectos adversos posiblemente estén asociadas con el uso de GLIADEL implante:
|
Durante los ensayos clínicos se observaron los siguientes efectos adversos en los pacientes, que fueron similares a los observados en pacientes sometidos a cirugía de tumor cerebral sin colocación de GLIADEL implantes.
Muy frecuentes: pueden afectar a más de 1 de cada 10 pacientes
- Trastornos psiquiátricos
Depresión
- Trastornos del sistema nervioso
Debilidad, especialmente de un lado del cuerpo; convulsiones (espasmos); confusión; dolor de cabeza, inflamación del cráneo; somnolencia; alteraciones del habla
- Trastornos vasculares
Inflamación vascular
- Trastornos gastrointestinales
Náuseas; vómitos; estreñimiento
- Trastornos de la piel y del tejido subcutáneo
Erupción cutánea; pérdida de cabello
- Trastornos renales y urinarios
Infección urinaria
- Trastornos generales y alteraciones en el lugar de administración
Empeoramiento del estado general; infección; dolor de cabeza; sensación de debilidad; fiebre o dolor, cicatrización anormal (lenta) de la herida quirúrgica.
Frecuentes: pueden afectar hasta 1 de cada 10 pacientes
- Trastornos de la sangre y del sistema linfático
Disminución de glóbulos rojos (anemia), lo que puede causar palidez cutánea, fatiga y ahogo; disminución de plaquetas sanguíneas, lo cual aumenta el riesgo de hemorragia; aumento de glóbulos blancos
- Trastornos endocrinos (problemas hormonales)
Diabetes mellitus
- Trastornos del metabolismo y de la nutrición
Edema periférico (exceso de líquido en los brazos o las piernas); baja concentración de sodio en la sangre, lo cual puede ocasionar fatiga y confusión, tics músculares, espasmos y coma; niveles altos de azúcar en la sangre, baja concentración de potasio en la sangre, lo cual puede ocasionar debilidad y tics musculares, y ritmo cardiaco anormal
- Trastornos psiquiátricos
Vambios en la personalidad, ansiedad excesiva, pensamiento anormal, alucinaciones, insomnio (poco sueño)
- Trastornos del sistema nervioso
Amnesia (pérdida de memoria); aumento de la tensión arterial intracraneal debido a un exceso de líquido; parálisis facial; falta de coordinación; sensibilidad reducida a la estimulación; sensaciones anormales de quemazón y picor; dificultad para caminar; mareos; crisis epiléptica (espasmos); temblores; meningitis; absceso (concentración localizada de pus); pérdida del conocimiento
- Trastornos oculares
Visión borrosa o anormal; inflamación del contorno de los ojos; dolor de ojos
- Trastornos vasculares
Sangrado, tensión sanguínea alta o baja
- Trastornos respiratorios, torácicos y mediastínicos
Infección pulmonar o pulmonía causante de ahogo, tos y fiebre
- Trastornos gastrointestinales
Infección microbiana de la boca; diarrea; estreñimiento; incontinencia fecal (pérdidas de heces incontroladas); dificultad para tragar; hemorragia gástrica o intestinal
- Trastornos epiteliales y subcutáneos
Erupción cutánea
- Trastornos musculoesqueléticos y del tejido conjuntivo
Infección general
- Trastornos renales y urinarios
Incontinencia urinaria, infecciones urinarias
- Trastornos generales y alteraciones en el lugar de administración
Dolor abdominal, desde la espalda hasta el pecho; inflamación facial; absceso (concentración localizada de pus); lesión accidental; reacción alérgica; dolor de cuello; infección sanguínea
Efectos adversos poco frecuentes (entre 1 y 10pacientes de cada 1000)
Lesiones traumáticas, intoxicaciones y complicaciones de procedimientos terapéuticos
Neumocefalia (acumulación de aire en la zona del implante)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto.
También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Gliadel implante
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en congelador a una temperatura de -20ºC o inferior.
Los sobres exteriores sin abrir pueden mantenerse a una temperatura menor de 22ºC durante un máximo de 6 horas. Pueden recongelarse una sola vez los sobres si no han sido abiertos y han permanecido por un máximo de 6 horas a una temperatura menor de 22º C. Después de la recongelación, el medicamento debe usarse en 30 días.
No utilice GLIADEL implante después de la fecha de caducidad que aparece en el envase y/o en el sobre después de CAD. La fecha de caducidad es el último día de ese mes que se indica. Su cirujano o el farmacéutico del hospital comprobarán la fecha de caducidad antes del uso de los implantes.
6. Contenido del envase e información adicional
Composición de GLIADEL implante
- El principio activo es carmustina. Cada implante contiene 7,7 mg. de carmustina
- El otro componente es polifeprosan 20.
Aspecto del producto y contenido del envase
GLIADEL implante está disponible en cajas de ocho láminas circulares implantables. Dichas láminas son implantes en forma de disco de color blanco opaco a amarillo. Cada implante está envasado individualmente en un sobre recubierto por una lámina de aluminio.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
CLINIGEN HEALTHCARE B.V.
Schiphol Boulevard 359
WTC Schiphol Airport, D Tower 11th floor
1118BJ Schiphol
Países Bajos
Responsable de la fabricación
ALMAC PHARMA SERVICES (IRELAND) LIMITED
Finnabair Industrial Estate
Dundalk
Co. Louth A91 P9KD
Irlanda
Tel: +353 42 932 0718
Fax: +353 42 932 0718
ALMAC PHARMA SERVICES LIMITED
20 Seagoe Industrial Estate
Craigavon, BT63 5QD,
Reino Unido
Tel: +44 (0)28 3836 3363
Fax: +44 (0)28 3836 3300
Fecha de la última revisión de este prospecto: 04/2021
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
PROSPECTO INFORMATIVO PARA PROFESIONALES SANITARIOS
- NOMBRE DEL MEDICAMENTO
GLIADEL 7,7 mg implante
- COMPOSICIÓN CUALITATIVA Y CUANTITATIVA
Cada implante contiene 7,7 mg de carmustina.
Para consultar la lista completa de excipientes, ver sección 6.1.
- FORMA FARMACÉUTICA
Implante.
Implante en forma de disco de color blanco opaco a amarillo.
- DATOS CLÍNICOS
4.1Indicaciones terapéuticas
GLIADEL implante está indicado para el tratamiento de pacientes adultos con glioma maligno de alto grado de nuevo diagnóstico como terapia adyuvante a la cirugía y radiación.
GLIADEL implante está indicado como terapia adyuvante a la cirugía para el tratamiento de pacientes con glioblastoma multiforme recurrente recidivante diagnosticado histológicamente, en los que esté indicada la resección quirúrgica.
4.2Posología y forma de administración
Posología
Vía intralesional sólo.
Cada implante de GLIADEL contiene 7,7 mg de carmustina, por lo que, tras la colocación de ocho implantes en la cavidad de resección del tumor, se libera una dosis de 61,6 mg.
Población pediátrica
No se han determinado la seguridad y la eficacia de GLIADEL implante en niños menores de 18 años. No hay datos disponibles.
Forma de administración
Se recomienda la colocación de un máximo de ocho implantes si el tamaño y la forma de la cavidad de resección lo permiten. Pueden utilizarse implantes seccionados por la mitad, pero los implantes fraccionados en más de dos partes deben ser eliminados en contenedores apropiados para desechos biopeligrosos (ver sección 6.6).
Se recomienda proceder a colocar los implantes directamente del envase interior estéril del producto a la cavidad de resección. Se pueden colocar apósitos de oxicelulosa sobre los implantes para fijarlos a la superficie de la cavidad (ver sección 6.6).
4.3Contraindicaciones
Hipersensibilidad al principio activo, o a alguno de los excipientes incluidos en la sección 6.1.
4.4Advertencias y precauciones especiales de empleo
Los pacientes sometidos a craneotomía e implantación de GLIADEL implante, deben ser monitorizados de forma estricta para detectar las posibles complicaciones de la craneotomía, que incluyen convulsiones, infecciones intracraneales, cicatrización anormal, edema cerebral y neumocefalia (ver sección 4.8). En pacientes tratados con GLIADEL implante se han descrito casos de efecto de masa intracerebral que no responde a los corticosteroides, incluido un caso que resultó en una hernia cerebral. En los pacientes tratados con GLIADEL implante debe monitorizarse rigurosamente la aparición de edema cerebral/hipertensión intracraneal tras la utilización de corticoides (ver sección 4.8). Las pérdidas de líquido cefalorraquídeo (LCR) fueron más frecuentes en pacientes tratados con GLIADEL implante. Se recomienda un cuidado especial para el cierre impermeable de la duramadre y en el tratamiento de las heridas locales (ver sección 4.8).
Se han descrito cambios en la pared de los vasos sanguíneos cerebrales ubicados cerca de la oblea de Gliadel, incluidos los casos de aneurisma que ocasionan sangrado cerebral varios meses después del implante de una oblea de Gliadel. Se debe evitar la implantación de obleas de Gliadel adyacente a grandes vasos cerebrales.
El desarrollo de edema cerebral con efecto de masa (debido a recidiva del tumor, infección intracraneal o necrosis) puede necesitar reintervención y, en algunos casos, retirada de GLIADEL implante o sus restos.
Se debe evitar la comunicación entre la cavidad de resección quirúrgica y el sistema ventricular para evitar que los implantes se desplacen al sistema ventricular y puedan producir hidrocefalia obstructiva. Si existe una vía de comunicación mayor que el diámetro del implante, ésta se debe cerrar previamente a la implantación de GLIADEL implante.
La tomografía computerizada y la resonancia magnética pueden mostrar una intensificación de la densidad del tejido cerebral que rodea la cavidad de resección después de la colocación de los implantes de GLIADEL. Este aumento de intensidad podría reflejar edema o inflamación causados por los implantes de GLIADEL, o progresión del tumor.
Las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos durante al menos 6 meses después de recibir GLIADEL implante.
Se debe aconsejar a los pacientes varones con parejas en edad fértil que utilicen métodos anticonceptivos efectivos durante al menos 90 días después de recibir GLIADEL implante
4.5Interacción con otros medicamentos y otras formas de interacción
Las interacciones de GLIADEL implante con otros fármacos o quimioterapia no han sido estudiadas formalmente.
4.6Fertilidad, embarazo y lactancia
Embarazo:
No existen estudios de GLIADEL implante en mujeres embarazadas, ni estudios que evalúen la toxicidad de GLIADEL implante sobre la reproducción.
Cuando se administra de forma sistémica, la carmustina, el principio activo de GLIADEL implante, puede tener efectos genotóxicos y afectar de forma negativa al desarrollo fetal (ver sección 5.3). Por lo tanto, no se recomienda utilizar GLIADEL implante durante el embarazo, ni en mujeres en edad fértil que no estén utilizando métodos anticonceptivos. Las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos durante al menos 6 meses después de recibir GLIADEL implante.
Se debe aconsejar a los pacientes varones con parejas en edad fértil que utilicen métodos anticonceptivos efectivos durante al menos 90 días después de recibir GLIADEL implante. No obstante, si aun así se considera necesario el uso de GLIADEL implante durante el embarazo, debe informarse a la paciente de los potenciales riesgos para el feto. En el caso de que la paciente se quede embarazada después de recibir GLIADEL implante, debe buscarse asesoramiento genético.
Lactancia:
Se desconoce si los componentes de GLIADEL implante se excretan por la leche materna. Dado que algunos fármacos se eliminan por leche materna y debido al posible riesgo de reacciones adversas graves de la carmustina en lactantes, está contraindicada la lactancia.
Fertilidad:
No se han llevado a cabo estudios de fertilidad con GLIADEL implante.
4.7Efectos sobre la capacidad para conducir y utilizar máquinas
GLIADEL implante no influye en la capacidad para conducir vehículos y utilizar maquinaria. Sin embargo, la crariotomía y GLIADEL implante pueden provocar alteraciones de la visión y del sistema nervioso. Por lo tanto el/la paciente debe conocer el efecto potencial de estas reacciones sobre la capacidad para conducir o utilizar máquinas.
4.8Reacciones adversas
El espectro de reacciones adversas observadas en pacientes con glioma maligno de alto grado de nuevo diagnóstico y gliomas malignos recidivantes fue consistente con el observado en pacientes sometidos a craneotomía por gliomas malignos.
Más abajo se enumeran las reacciones adversas muy frecuentes (≥ 1/10), frecuentes (≥1/100 a <1/10) e infrecuentes (≥1/1000 a <1/100) observadas en los pacientes tratados con GLIADEL implante durante los ensayos clínicos.
Las reacciones adversas se enumeran en orden decreciente de gravedad dentro de cada frecuencia.
Cirugía Primaria
Los siguientes datos corresponden a las reacciones adversas más frecuentes observadas en el 5% o más de los 120 pacientes con glioma maligno de nuevo diagnóstico tratados con GLIADEL implante en el ensayo clínico.
Reacciones adversas frecuentes observadas en≥5% de los pacientes tratados con GLIADEL implante en la cirugía inicial
Clasificación por órganos y sistemas | Reacciones adversas | |
Trastornos endocrinos | frecuentes* | Diabetes mellitus |
Trastornos psiquiátricos | muy frecuentes | Depresión |
frecuentes | Trastorno de la personalidad, ansiedad, pensamiento anormal, alucinaciones, insomnio | |
Trastornos del sistema nervioso | muy frecuentes | Hemiplejia, convulsiones, confusión, edema cerebral, afasia, somnolencia, alteraciones del habla |
frecuentes | Amnesia, aumento de presión intracraneal, trastornos de la personalidad, ansiedad, parálisis facial, neuropatía, ataxia, hipoestesia, parestesia, pensamiento anormal, deambulación anormal, mareo, convulsiones tipo “grand mal”, alucinaciones, insomnio, temblores | |
Trastornos oculares | frecuentes | Edema conjuntival, visión anormal, alteraciones del campo visual |
Trastornos vasculares | muy frecuentes | Tromboflebitis |
frecuentes | Hemorragia | |
Trastornos respiratorios, torácicos y mediastínicos | frecuentes | Embolia pulmonar |
Infecciones e infestaciones Trastornos gastrointestinales | frecuentes | Neumonía |
muy frecuentes | Náuseas, vómitos, estreñimiento | |
Trastornos de la piel y del tejido subcutáneo | frecuentes | Diarrea |
Trastornos de la piel y del tejido subcutáneo | muy frecuentes | Erupción cutánea, alopecia |
Trastornos renales y urinarios | frecuentes | Infección del tracto urinario, incontinencia urinaria |
Trastornos generales y alteraciones en el lugar de administración | muy frecuentes | Empeoramiento, cefalea , astenia, infección, fiebre, dolor, cicatrización anormal |
frecuentes | Dolor abdominal, dolor de espalda, edema facial, dolor torácico, absceso, lesión accidental, edema periférico |
Se notificó hipertensión intracraneal más frecuentemente en los pacientes tratados con GLIADEL implante que en los que recibieron placebo (9,2% frente a 1,7%), siendo un hallazgo tardío coincidente con la recidiva del tumor, y se consideró poco probable su asociación con el uso de GLIADEL implante (ver sección 4.4).
Las pérdidas de LCR fueron más frecuentes en pacientes tratados con GLIADEL implante que en pacientes que recibieron placebo. Sin embargo, no aumentaron las infecciones intracraneales ni otras anomalías de cicatrización (ver sección 4.4).
Cirugía en enfermedad recidivante
Se observaron las siguientes reacciones adversas después de la operación en el 4% o más de los pacientes tratados con GLIADEL implante en cirugía repetida. Sólo se listan las reacciones que fueron más frecuentes en el grupo que recibió GLIADEL implante que en el grupo placebo, con la excepción de los efectos sobre el sistema nervioso, de los que los implantes placebo podrían ser la causa. Estas reacciones adversas, o bien no estaban presentes antes de la operación, o bien empeoraron después de la operación durante el periodo de seguimiento. El periodo de seguimiento fue de hasta 71 meses.
Reacciones adversas frecuentes observadas en ≥ 4% de los pacientes tratados con GLIADEL implante en la cirugía repetida
Clasificación por órganos y sistemas | Reacciones adversas | |
Trastornos de la sangre y del sistema linfático | frecuentes | Anemia |
Trastornos del metabolismo y de la nutrición | frecuentes | Hiponatremia |
Trastornos del sistema nervioso | muy frecuentes | Convulsión, hemiplejia, cefalea, somnolencia, confusión |
frecuentes | Afasia, estupor, edema cerebral, aumento de la presión intracraneal, meningitis o absceso | |
Trastornos vasculares | frecuentes | Tromboflebitis |
Trastornos respiratorios, torácicos y mediastínicos | frecuentes | Embolia pulmonar |
Trastornos gastrointestinales | frecuentes | Náuseas, vómitos |
Trastornos de la piel y del tejido subcutáneo | frecuentes | Erupción cutánea |
Trastornos renales y urinarios | muy frecuentes | Infección del tracto urinario |
Trastornos generales y alteraciones en el lugar de administración | muy frecuentes | Cicatrización anormal |
frecuentes | Infección, dolor |
Los siguientes efectos adversos, los cuales no están incluidos en la tabla a continuación, se notificaron en pacientes tratados con implante GLIADEL en todos los estudios. Los efectos enumerados o bien no estaban presentes durante el período previo a la cirugía, o bien empeoraron en el período posterior a la cirugía. No fue posible establecer si el implante GLIADEL fue la causa de estos efectos
Efectos adversos en pacientes que reciben implante de GLIADEL
Clasificación por órganos y sistemas | Reacciones adversas | |
Trastornos de la sangre y del sistema linfático | frecuentes | Trombocitopenia, leucocitosis |
Trastornos del metabolismo y de la nutrición | frecuentes | Hiponatremia, hiperglucemia, hipopotasemia |
Trastornos del sistema nervioso | frecuentes | Hidrocefalia, ataxia, mareo, hemiplejia, coma, amnesia, diplopia, |
poco frecuentes | Hemorragia cerebral, infarto cerebral | |
Trastornos psiquiátricos | frecuentes | Depresión, pensamiento anormal, insomnio, reacción paranoide |
Trastornos oculares | frecuentes | Alteración visual, dolor ocular |
Trastornos cardiacos y vasculares | frecuentes | Hipertensión, hipotensión |
Trastornos respiratorios, torácicos y mediastínicos | frecuentes | Infección, neumonía por aspiración |
Trastornos gastrointestinales | frecuentes | Diarrea, estreñimiento, disfagia, hemorragia gastrointestinal, incontinencia fecal |
Trastornos de la piel y del tejido subcutáneo | frecuentes | Erupción cutánea |
Trastornos musculoesqueléticos y del tejido conjuntivo | frecuentes | Infección |
Trastornos renales y urinarios | frecuentes | Incontinencia urinaria |
Trastornos generales y alteraciones en el lugar de administración | frecuentes | Edema periférico, dolor de cuello, lesión accidental, dolor de espalda, reacción alérgica, astenia, dolor torácico, sepsis |
Lesiones traumáticas, intoxicaciones y complicaciones de procedimientos terapéuticos | Poco frecuentes | neumocefalia |
Se han notificado casos de acumulación de aire en la zona del implante con Gliadel, en ocasiones asociados con síntomas neurológicos (hemiplejia, afasia, convulsiones).
Las siguientes cuatro categorías de reacciones adversas están posiblemente relacionadas con el tratamiento con GLIADEL implante.
Convulsiones:
En el ensayo clínico de cirugía inicial, la incidencia de convulsiones en los cinco primeros días después de la implantación fue del 2,5% en el grupo GLIADEL implante.
En el ensayo clínico de cirugía en enfermedad recidivante, la incidencia de convulsiones después de la operación fue 19% en pacientes tratados con GLIADEL implante. En este ensayo, 12/22 (54%) de los pacientes tratados con GLIADEL implante experimentaron el inicio de las convulsiones o el empeoramiento de las existentes durante los cinco primeros días después de la operación. La mediana del tiempo hasta el inicio de las convulsiones o el empeoramiento de las existentes después de la operación fue 3,5 días en los pacientes tratados con GLIADEL implante.
Edema cerebral:
El desarrollo de un edema cerebral con efecto de masa (debido a recidiva del tumor, infección intracraneal o necrosis) puede obligar a una reintervención y, en algunos casos, a la retirada de GLIADEL implante o sus restos (ver sección 4.4).
Anomalías en la cicatrización:
Se han registrado las siguientes anomalías en la cicatrización en ensayos clínicos de GLIADEL implante: dehiscencia de la herida, retraso en la cicatrización de la herida, efusiones subdurales, subgaleales o a través de la sutura, y pérdidas de líquido cefalorraquídeo.
En el ensayo realizado en cirugía inicial, las pérdidas de líquido cefalorraquídeo ocurrieron en un 5% de las personas que recibieron GLIADEL implante. Durante la cirugía, se debe asegurar un cierre impermeable de la duramadre para minimizar el riesgo de pérdida de líquido cefalorraquídeo (ver sección 4.4).
Infección intracraneal:
En el ensayo clínico en cirugía inicial, la incidencia de absceso cerebral o meningitis fue del 5% en pacientes tratados con GLIADEL implante.
En cirugía de la enfermedad recidivante, la incidencia de absceso cerebral o meningitis fue del 4% en pacientes tratados con GLIADEL implante.
En un ensayo clínico publicado se ha notificado la formación de quistes después del tratamiento con GLIADEL implante. Esta reacción ocurrió en el 10% de los pacientes observados en el ensayo. Sin embargo, la formación de quistes es posible tras la resección de un glioma maligno.
Notificación de sospecha de reacciones adversas
Es importante notificar las sospechas de reacciones adversas al medicamento tras su autorización. Ello permite una supervisión continuada de la relación beneficio/riesgo del medicamento. Se invita a los profesionales sanitarios a notificar las sospechas de reacciones adversas a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es.
4.9Sobredosis
No procede.
- PROPIEDADES FARMACOLÓGICAS
5.1Propiedades farmacodinámicas
Grupo farmacoterapéutico: Agentes antineoplásicos, agentes alquilantes, nitrosoureas, código ATC: L01AD01
Datos preclínicos
GLIADEL implante libera carmustina directamente en la cavidad quirúrgica creada después de la resección tumoral. Al ser expuestos al medio acuoso de la cavidad, los enlaces anhídrido del copolímero son hidrolizados, liberando carmustina, carboxifenoxipropano y ácido sebácico. La carmustina liberada de GLIADEL implante difunde al tejido cerebral circundante y ejerce un efecto antineoplásico mediante la alquilación del ADN y ARN.
La carmustina se degrada y metaboliza de forma espontánea, creándose el grupo alquilante, presuntamente un ión carbonio cloroetilo, que conduce a la formación irreversible de enlaces cruzados (cross-linking) en el ADN.
La actividad antitumoral de GLIADEL implante depende de la liberación de carmustina en la cavidad tumoral en cantidades suficientes para una citotoxicidad eficaz.
En 3 semanas se degrada más del 70% del copolímero. Los monómeros tienen diferente metabolismo y eliminación. El carboxifenoxipropano se elimina mayoritariamente por los riñones, y el ácido sebácico, un ácido graso endógeno, es metabolizado por el hígado y se espira en forma de CO2 en animales.
Eficacia clínica y seguridad
Cirugía inicial
En un ensayo clínico aleatorizado, doble ciego, controlado con placebo, en 240 adultos con glioma maligno de alto grado de nuevo diagnóstico sometidos a craneotomía inicial para resección del tumor, la mediana de la supervivencia aumentó de 11,6 meses con placebo a 13,9 meses con GLIADEL implante (p = 0,079, log-rank testno estratificado) en la fase inicial de seguimiento del ensayo. El tipo de tumor más común fue el glioblastoma multiforme (GBM) (n=207), seguido de oligoastrocitoma anaplásico (n=11), oligodendroglioma (n=11), y astrocitoma anaplásico (n=2). El índice de riesgos (hazard ratio) para GLIADEL implante fue de 0,77 (95% IC: 0,57 – 1,03). En la fase de seguimiento a largo plazo, los pacientes que aún seguían vivos al final de la fase inicial de seguimiento fueron seguidos hasta tres años como mínimo o hasta su muerte. La mediana de la supervivencia aumentó de 11,6 meses con placebo a 13,9 meses con GLIADEL implante (p < 0,05, log-rank test). El índice de riesgos (hazard ratio) del tratamiento con GLIADEL implante fue de 0,73 (95% IC: 0,56 – 0,95).
Cirugía en enfermedad recidivante
En un ensayo clínico aleatorizado, doble ciego, controlado con placebo, en 145 adultos con glioblastoma (GBM) recidivante, GLIADEL implante prolongó la supervivencia de estos pacientes. El 95% de los pacientes tratados con GLIADEL implante recibió de 7 a 8 implantes.
La tasa de supervivencia con placebo a los 6 meses fue del 36% (26/73), frente a un 56% (40/72) con el tratamiento con GLIADEL implante. La mediana de la supervivencia de los pacientes con GBM fue de 20 semanas con placebo, frente a 28 semanas con el tratamiento de GLIADEL implante.
5.2Propiedades farmacocinéticas
No se conoce la absorción, distribución, metabolismo ni eliminación del copolímero en humanos. Las concentraciones de carmustina liberadas por GLIADEL implante en el tejido cerebral humano no han sido determinadas. No es posible determinar los niveles plasmáticos de carmustina después de la implantación de GLIADEL implante. La carmustina no se detecta en sangre o líquido cefalorraquídeo, en conejos con implantes de carmustina al 3,85%.
Después de una perfusión intravenosa de carmustina con dosis entre 30 y 170 mg/m2, la media de la semivida de eliminación terminal, del aclaramiento y del volumen de distribución en estado estacionario son, respectivamente, 22 minutos, 56 ml/min/kg y 3,25 l/kg. Aproximadamente, el 60% de una dosis intravenosa de 200 mg/m2 de 14C-carmustina se excreta por orina en 96 horas y el 6% se espira en forma de CO2.
Los implantes de GLIADEL son biodegradables en el cerebro humano cuando son colocados en la cavidad tras la resección del tumor. La velocidad de biodegradación varía entre paciente y paciente. Durante el proceso de biodegradación se pueden observar restos de implantes mediante técnicas de imagen cerebral o en la siguiente operación, incluso aunque se haya producido una amplia degradación de todos los componentes.
5.3Datos preclínicos sobre seguridad
No se han llevado a cabo estudios de carcinogenicidad, mutagenicidad, toxicidad embrio-fetal, toxicidad pre- y postnatal, y alteración de la fertilidad con GLIADEL implante.
La carmustina, el principio activo de los implantes de GLIADEL, cuando se administra sistémicamente, tiene efectos embriotóxicos, teratógenos, genotóxicos y carcinogénicos y puede causar degeneración testicular en varios modelos animales.
- DATOS FARMACÉUTICOS
6.1Lista de excipientes
Polifeprosan 20
6.2Incompatibilidades
No procede.
6.3Periodo de validez
4 años.
6.4Precauciones especiales de conservación
Conservar en congelador. No conservar a temperatura superior a -20º C.
Los sobres exteriores sin abrir pueden mantenerse a una temperatura menor de 22º C durante un máximo de 6 horas.
El medicamento puede ser recongelado una sola vez si los sobres no han sido abiertos y han permanecido por un máximo de 6 horas a una temperatura menor de 22º C. Después de la recongelación, el medicamento debe usarse en 30 días.
6.5Naturaleza y contenido delenvase
GLIADEL implante está disponible en una caja que contiene 8 implantes. Cada implante está acondicionado de forma individual en 2 sobres de aluminio laminado.
6.6Precauciones especiales de eliminacióny otras manipulaciones
Los implantes deben ser manipulados por personal provisto de guantes quirúrgicos, ya que la exposición a la carmustina puede ocasionar quemaduras graves e hiperpigmentación de la piel. Se recomienda el uso de guantes dobles y, después de su uso, los guantes externos deben ser desechados en un contenedor de productos biopeligrosos. Durante la colocación de los implantes debe emplearse un instrumento quirúrgico reservado a la manipulación de los implantes. Si está indicada la repetición de la intervención neuroquirúrgica, cualquier implante o resto de implante debe manipularse como un posible agente citotóxico. La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local para agentes citotóxicos.
Los implantes de GLIADEL deben ser manipulados con precaución. Los sobres que contienen los implantes de GLIADEL deben ser entregados en la sala de operaciones y deben permanecer cerrados hasta el momento de la colocación de los implantes en la cavidad de resección. Únicamente la superficie externa del sobre exterior no es estéril. En cualquier caso, si se deja caer accidentalmente un implante, debe ser desechado adecuadamente.
Instrucciones para abrir los sobres que contienen el implante:
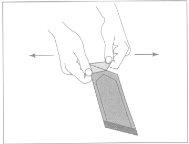
Figura1:Para abrir el sobre externo,
localizar la esquina doblada y tirar suavemente hacia fuera.
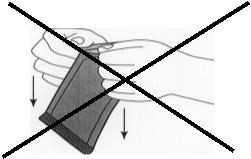
Figura 2:No tirar hacia abajo girando los nudillos
en el sobre. Ello podría ejercer presión sobre el implante
y romperlo.
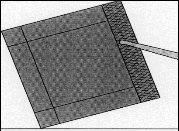
Figura 3:Retirar el sobre interior con la ayuda
de unas pinzas y tirando hacia arriba.
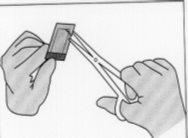
Figura 4:Para abrir el sobre interior, sostenerlo suavemente
y cortarlo circularmente alrededor del implante.
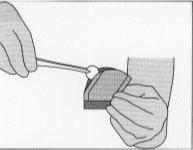
Figura 5:Para retirar el implante, cogerlo suavemente
con la ayuda de unas pinzas y colocarlo en la cavidad de resección.
En cualquier caso, si se deja caer un implante, debe ser desechado adecuadamente.
Una vez que se ha realizado la resección del tumor, que el diagnóstico del tumor ha sido confirmado por anatomía patológica y se ha conseguido la hemostasia, se pueden colocar hasta 8 implantes para cubrir lo más posible la cavidad de resección. Se considera aceptable una ligera superposición entre los implantes. Pueden utilizarse implantes seccionados por la mitad, pero si están fragmentados en más de dos partes, deben ser eliminados en contenedores de desechos biopeligrosos.
Pueden colocarse tiras de apósito de oxicelulosa sobre los implantes para fijarlos a la superficie de la cavidad. Después de la colocación de los implantes, la cavidad de resección debe estar irrigada y la duramadre debe cerrarse de forma impermeable.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local para residuos biopeligrosos.
- TITULAR DE LA AUTORIZACIÓN DE COMERCIALIZACIÓN
CLINIGEN HEALTHCARE B.V.
Schiphol Boulevard 359
WTC Schiphol Airport, D Tower 11th floor
1118BJ Schiphol
Países Bajos
- NÚMERO(S) DE AUTORIZACIÓN DE COMERCIALIZACIÓN
Reg. A.E.M.P.S. n° 62.745
- FECHA DE LA PRIMERA AUTORIZACIÓN/RENOVACIÓN DE LA AUTORIZACIÓN
Fecha de la primera autorización: 19/10/1999
Fecha de la última renovación: 10/12/2008
- FECHA DE LA REVISIÓN DEL TEXTO
Abril 2021
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GLIADEL 7,7 mg IMPLANTEForma farmacéutica: INYECTABLE PERFUSION, 100 mgPrincipio activo: CarmustinaFabricante: Zentiva K.S.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 mgPrincipio activo: CarmustinaFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 300 mgPrincipio activo: CarmustinaFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para GLIADEL 7,7 mg IMPLANTE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GLIADEL 7,7 mg IMPLANTE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






